
AllCancer 4D Cancer Metabolic Therapy: Pioneering Cancer Teartment for a Cure in Hong Kong
Central to the company’s approach is its groundbreaking “International Four-Dimensional (4D) Therapy” platform, redefining traditional cancer treatment paradigms. This advanced framework comprises four sophisticated therapeutic dimensions designed to attack cancer comprehensively:
- Metabolic Reprogramming: Altering cancer cell metabolism pathways to starve tumors of necessary nutrients and energy, effectively inhibiting their proliferation.
- Dual Immune Modulation: Implementing a dual-strategy immune modulation that enhances the body’s anti- tumor immune response while simultaneously reducing immune suppression, optimizing immune system effectiveness against cancer.
- Smart Nano-Targeting: Utilizing advanced nanotechnology for precision drug delivery directly to cancer cells, maximizing therapeutic effectiveness and minimizing systemic side effects.
- Tumor Microenvironment Remodeling: Modifying the tumor’s surrounding environment to decrease tumer viability, normalize vasculature, reduce immunosuppressive factors, and enhance the penetration of therapeutic agents and immune cells.
By integrating these four technological pillars, International 4D Therapy provides a comprehensive, innovative approach that surpasses traditional cancer therapies.

Global Patent Protection and Leadership
The proprietary technologies underpinning the 4D Therapy platform are protected by extensive international patents granted in China, the United States, Europe, and Japan. This global patent portfolio ensures a robust technological advantage, positioning All Cancer Medical Solutions Limited at the forefront of oncology innovation. With these globally safeguarded therapeutic advances and a distinguished multidisciplinary team, the company is uniquely positioned as a leader in next-generation cancer therapeutics, committed to delivering sophisticated, effective, and personalized patient care.
Can Cancer Be Cured?
Cancer’s curability depends on its type, stage, and treatment. Some cancers, like early-stage breast or prostate cancer, can often be cured with surgery, radiation, or chemotherapy, achieving long-term remission. However, a universal cure for all cancers doesn’t exist, and recurrence remains a risk. Advanced stages or aggressive cancers, like pancreatic, are less likely to be cured. Ongoing research improves outcomes, but cancer is complex, with over 200 types.
Source: American Cancer Society (2021).
https://www.cancer.org/cancer/understanding-cancer/can-cancer-be-cured.html
Source: American Cancer Society (2021).
https://www.cancer.org/cancer/understanding-cancer/can-cancer-be-cured.html

Advanced Oncology Therapeutic Methodologies
Metabolic Reprogramming
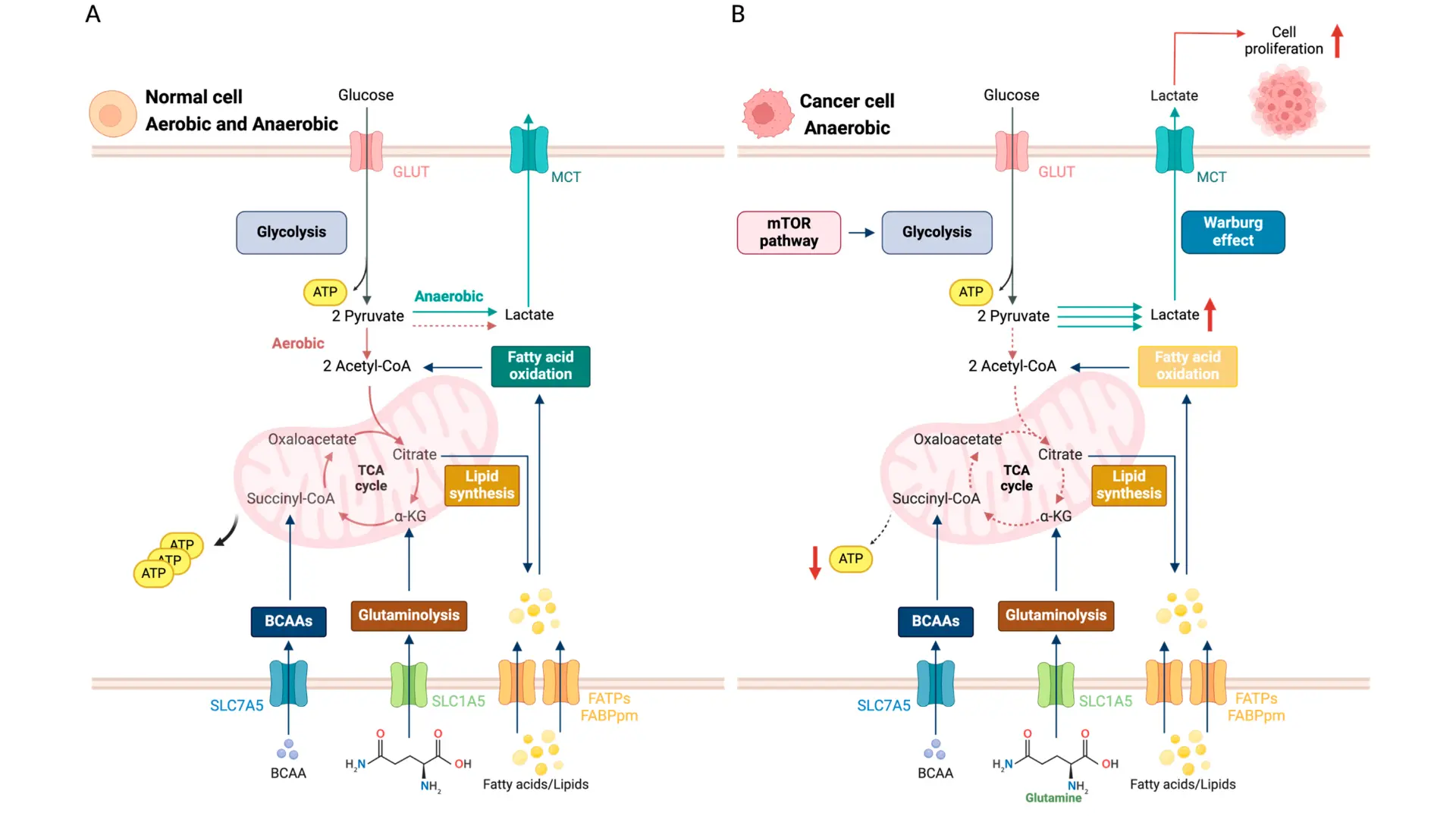
- Underlying Mechanisms Cancer cells alter their metabolism to meet the high demands of rapid growth and survival. They commonly exhibit the Warburg effect, relying on aerobic glycolysis (high glucose uptake and lactate production even in oxygen) instead of efficient mitochondrial respiration . Additionally, tumors upregulate other pathways like glutaminolysis (dependence on glutamine) and enhanced fatty acid synthesis to supply building blocks and energy for proliferation . These metabolic shifts are driven by oncogenes (e.g. MYC, RAS) and loss of tumor suppressors, which rewire cellular pathways to favor nutrient uptake and biosynthesis, helping cancer cells thrive in nutrient-poor, hypoxic environments . In essence, the tumor is “reprogrammed” to fuel itself at the molecular level, creating unique metabolic vulnerabilities.
- Clinical Applications Therapies targeting these metabolic vulnerabilities are emerging in cancer treatment. For example, inhibitors of key glycolytic enzymes (hexokinase, LDH) and glutamine analogs are in development to starve tumors of critical fuels . Some metabolic drugs have reached the clinic: IDH1/2 enzyme inhibitors are used in IDH-mutant leukemias and gliomas, directly countering an aberrant metabolic enzyme and leading to tumor responses . Another established approach is exploiting amino acid dependence – L-asparaginase, for instance, is routinely used in leukemia to deplete asparagine and selectively kill cancer cells. Even dietary interventions (such as ketogenic diets or fasting-mimicking diets) are being explored to complement therapy by limiting nutrients available to the tumor . Collectively, these strategies “starve” the cancer or disrupt its energy production, applying a precise metabolic pressure against tumor cells.
- Advantages Targeting cancer metabolism offers several benefits over conventional chemotherapy. First, it can be highly selective – normal cells are generally more metabolically flexible and can use alternate pathways, whereas cancer cells stuck in their reprogrammed state are more susceptible to metabolic inhibitors . This selectivity means metabolic therapies can kill tumor cells while sparing healthy tissue, translating to lower systemic toxicity for patients . Second, attacking the tumor’s energy and building-block supply line can overcome resistance to standard therapies: even when cancer cells mutate to evade drugs, they cannot easily escape fundamental metabolic needs . In fact, combining metabolic therapies with targeted drugs or immunotherapy has been shown to improve efficacy by cutting off the tumor’s adaptive “fuel” that drives treatment resistance . Overall, metabolic reprogramming treatments add a powerful, mechanism-based modality that complements existing therapies, aiming to suffocate tumors from within while minimizing harm to normal cells
Dual Immune Modulation
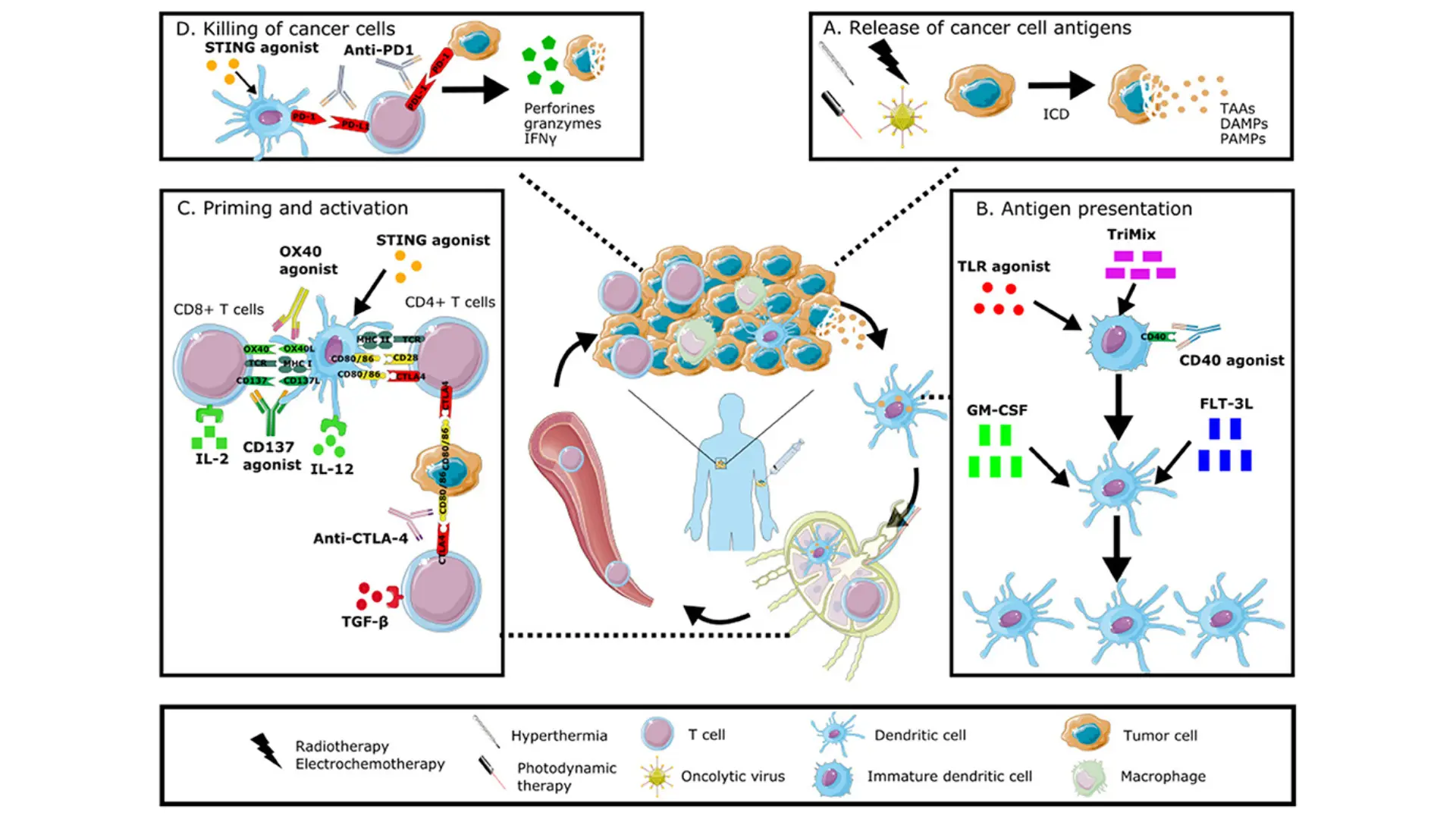
- Underlying Mechanisms Dual immune modulation involves simultaneously targeting two distinct immunoregulatory pathways to amplify the body’s anti-tumor immune response. At the cellular level, tumors evade immunity through multiple “checkpoints” and suppressive signals; dual modulation tackles two of these mechanisms at once. A prime example is dual checkpoint inhibition: blocking PD-1/PD-L1 and CTLA-4 together releases two brakes on T cells, unleashing a more potent attack on cancer than targeting either alone . Other dual approaches pair an immune checkpoint blocker with an immune activator – for instance, bispecific antibodies are being designed to both disable an inhibitory receptor (like TIGIT) and stimulate a co-stimulatory receptor (like 4-1BB) on T cells, thereby removing inhibition while providing a “go” signal to immune cells . In essence, dual immune modulation works at the molecular level by enhancing cytotoxic immune cell activity (e.g. CD8⁺ T cells, NK cells) and/or mitigating immunosuppressive factors in the tumor microenvironment in parallel. This two-pronged strategy creates a more robust immune assault on cancer cells than single-pathway immunotherapy.
- Clinical Applications Dual immune-modulating strategies are already being applied in oncology. A notable success is the combination of nivolumab (PD-1 inhibitor) with ipilimumab (CTLA-4 inhibitor), which has become an approved treatment in melanoma, lung cancer, renal carcinoma and other cancers. Clinically, this dual checkpoint blockade has yielded higher tumor response rates and longer survival compared to using either agent alone . For example, in trials for advanced liver cancer, patients receiving dual therapy showed improved outcomes – the CheckMate-040 study in hepatocellular carcinoma demonstrated enhanced tumor regression with PD-1 + CTLA-4 blockade versus historical single-agent therapy . Another real-world example is combining PD-L1 immunotherapy with anti-VEGF angiogenesis inhibition: the regimen of atezolizumab (anti-PD-L1) plus bevacizumab (anti-VEGF) in liver cancer achieves dual immune modulation by both reinvigorating T cells and normalizing the tumor vasculature, leading to significantly better overall survival than standard treatment (median 19.2 vs. 13.4 months) . Beyond checkpoints, new bi-functional biologics (like bispecific T cell engagers and dual-action cytokines) are in trials, aiming to concurrently direct immune cells to tumors and activate them. These applications highlight the versatility of dual modulation – whether it’s two checkpoints or an immune-checkpoint plus another pathway, the goal is a synergistic immune onslaught on cancer.
- Advantages Dual immune modulation provides enhanced efficacy and broader anti-cancer activity compared to traditional single-agent immunotherapies or chemotherapy. By blocking two immune escape routes at once, tumors have a harder time evading the immune system – this often translates into deeper and more durable tumor responses . Patients treated with dual checkpoint inhibitors, for instance, have achieved higher remission rates in diseases like metastatic melanoma, including complete responses in a subset of cases that were refractory to standard chemotherapy. Another advantage is the synergy observed: one agent can potentiate the effect of the other. In the PD-1 plus CTLA-4 example, CTLA-4 blockade expands the pool of T cells while PD-1 blockade sustains their activity – together they generate a more robust T-cell attack than either alone, overcoming resistance mechanisms that might hinder a single therapy. Clinically, this synergy has led to improved survival, as seen with the PD-L1/VEGF inhibition combo which leverages immune activation plus a favorable tumor microenvironment change . Importantly, unlike non-specific treatments (e.g. chemotherapy), dual immunotherapy specifically targets immune pathways, so it can produce lasting anti-tumor immunity (immune memory) that keeps cancers at bay long after treatment. In summary, dual immune modulation amplifies the body’s natural defenses on two fronts, offering a more powerful and precise approach than conventional therapy and achieving outcomes that were not possible with single-modality treatment
Smart Nano-Targeting
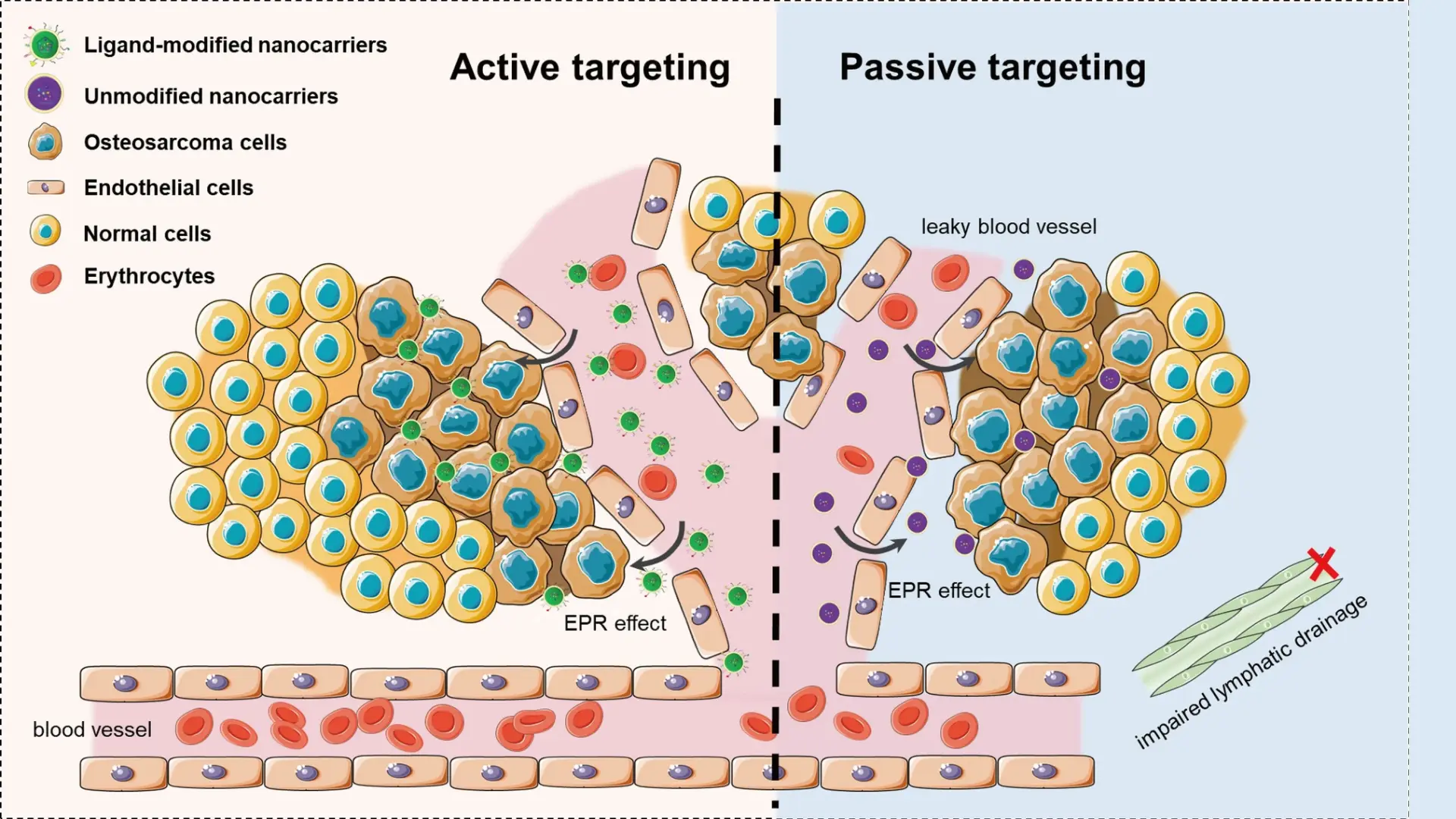
- Underlying Mechanisms Smart nano-targeting employs nanoparticle-based carriers (typically 10–100 nm in size) that can precisely deliver therapeutic agents to cancer cells while sparing normal tissue. These “smart” nanoparticles are engineered with features that make them selectively accumulate in tumors and release their payload in a controlled manner. One mechanism is active targeting: nanoparticles are functionalized with ligands (such as antibodies, peptides, or aptamers) that bind to receptors overexpressed on cancer cells, homing in on tumors like a guided missile . Another mechanism is stimuli-responsive release: the nanocarrier is designed to sense the tumor’s microenvironment (for example, the acidic pH, certain enzymes, or higher temperature in tumors) and only unload the drug under those conditions . Some nanoparticles even respond to external triggers (like light, ultrasound, or magnetic fields) to induce drug release at the tumor site . At the cellular level, these nanoparticles can enter tumor tissue via the leaky blood vessels that supply tumors (the enhanced permeability and retention (EPR) effect) and then bind or be taken up by cancer cells, depositing high concentrations of the drug directly inside or around malignant cells . Thus, smart nano-targeting works through a combination of precision targeting of tumor markers and conditional drug release, achieving a high local drug effect in cancer cells while reducing exposure elsewhere.
- Clinical Applications Nanotechnology-based cancer therapies have already made significant inroads in treatment. A well-known example is liposomal chemotherapy – drugs like doxorubicin have been encapsulated in liposome nanoparticles (e.g. Doxil) to treat breast and ovarian cancers. The liposomes preferentially accumulate in tumor tissue via passive targeting (EPR effect), which increases the drug concentration in the tumor and reduces heart toxicity compared to conventional doxorubicin . Another approved nanomedicine is nab-paclitaxel (Abraxane), where paclitaxel is bound to albumin nanoparticles; this formulation improves solubility and tumor uptake of the drug in pancreatic and breast cancers. Researchers are also developing polymer-based and metallic nanoparticles that carry chemotherapy, gene-silencing RNA, or even CRISPR gene editors directly to cancer cells. These can be engineered to release their payload when they encounter specific tumor stimuli – for instance, pH-sensitive nanoparticles that dissolve and release drug in the acidic tumor milieu, or heat-triggered gold nanoshells that, upon near-infrared light exposure, release a thermal dose to ablate tumor cells . “Smart” nanocarriers are being explored for combination theranostics as well: some designs allow imaging and therapy together (so doctors can track drug delivery in real time). In immunotherapy, nanoparticle vaccines and nano-adjuvants are under study to better deliver tumor antigens to immune cells . These applications demonstrate how smart nano-targeting is already translating into real treatments, achieving drug delivery with unprecedented precision.
- Advantages Smart nano-targeting offers greater precision and safety than traditional cancer therapies like systemic chemotherapy. By concentrating therapeutic agents at the tumor site, nanocarriers minimize collateral damage to healthy tissues – this means patients experience fewer side effects (for example, less nausea, hair loss, or organ toxicity) because the drug is not bathing the entire body at high doses . The improved pharmacokinetics (longer circulation of the drug and higher tumor uptake) effectively raises the therapeutic index of anticancer drugs . Another major advantage is the ability of nanotechnology to overcome drug resistance mechanisms. Nanoparticles can be engineered to bypass multidrug efflux pumps or to co-deliver synergistic drugs, tackling resistance at the tumor site. In fact, nanotech-enabled therapies have been shown to resensitize tumors that no longer responded to standard chemo by ensuring the drug reaches its target in the cancer cell at sufficient concentrations . Moreover, smart nanoparticles enable novel types of therapies: they can ferry delicate molecules (like siRNA, mRNA, or proteins) that would be rapidly degraded or cleared if given normally, thus opening doors to gene therapy and advanced immunotherapies in cancer . The multi-functionality of these platforms (combining targeting, imaging, and multi-drug delivery) can lead to more effective treatment regimens – for instance, simultaneously attacking a tumor on multiple fronts or monitoring response in real time. In summary, smart nano-targeting provides a leap in precision medicine for oncology – it delivers potent therapy exactly where needed, boosting efficacy while reducing systemic toxicity, and it creates opportunities to treat cancers in ways conventional therapies simply could not achieve .
Tumor Microenvironment Remodeling
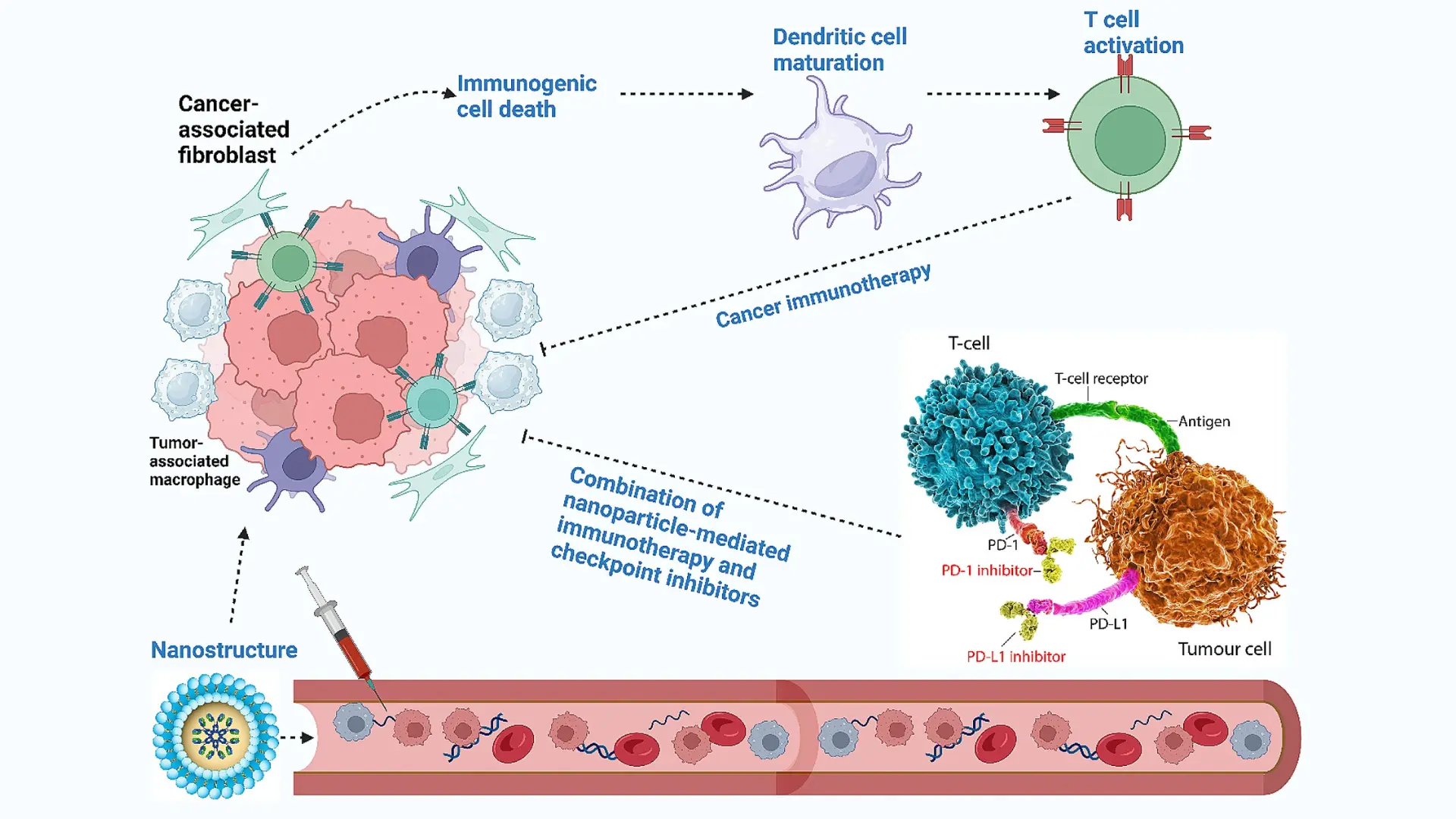
- Underlying Mechanisms Tumor microenvironment (TME) remodeling refers to therapeutic strategies that actively alter the tumor’s surrounding environment – which includes immune cells, blood vessels, fibroblasts, extracellular matrix, and signaling molecules – to hinder cancer growth and enhance treatment responses. At the molecular and cellular level, this can involve several approaches. One key mechanism is normalizing abnormal tumor vasculature: tumors often have disorganized, leaky blood vessels that cause hypoxia and prevent immune cell infiltration. Using agents like VEGF inhibitors, it is possible to prune and stabilize these vessels, improving oxygen delivery and perfusion. This vascular “normalization” not only starves the tumor by cutting off excess blood supply but also reprograms the microenvironment – oxygenation is increased and immune cells can penetrate the tumor better, shifting the TME from immunosuppressive to immunosupportive . Another mechanism is repolarizing immune cells in the TME. Tumors often exploit immune suppressor cells (such as M2-polarized tumor-associated macrophages, Tregs, and myeloid-derived suppressor cells). TME remodeling therapies might block the signals (e.g. CSF-1, TGF-β) that recruit or activate these suppressive cells, thereby reducing their numbers and/or converting them to pro-inflammatory, tumor-fighting phenotypes . Similarly, targeting fibroblast activity or enzymatically degrading dense extracellular matrix can relieve physical barriers to therapy. In short, TME remodeling works by converting a “pro-tumor” niche into an “anti-tumor” one – by normalizing blood flow, removing immunosuppressive factors, and enhancing immune and drug access to cancer cells.
- Clinical Applications Therapies that remodel the tumor microenvironment are being integrated into cancer treatment with promising results. A prominent example is anti-angiogenic therapy. Drugs like bevacizumab (a VEGF-neutralizing antibody) are used in cancers such as colorectal, ovarian, and glioblastoma to target the tumor vasculature. Bevacizumab not only prunes blood vessels (limiting the tumor’s nutrient supply) but also has been shown to normalize remaining vessels and improve the efficacy of other treatments. In clinical studies, combining bevacizumab with immunotherapy or chemotherapy has yielded superior outcomes – for instance, in ovarian cancer and hepatocellular carcinoma, anti-VEGF plus checkpoint inhibitor therapy led to higher response rates and longer survival than either alone . Another application of TME remodeling is targeting immunosuppressive cytokines: TGF-β inhibitors are in trials for cancers like liver and lung; in preclinical models of hepatocellular carcinoma, blocking TGF-β signaling reversed immune suppression – it reduced Treg cell infiltration and enabled a robust CD8⁺ T-cell attack, causing regression of otherwise resistant tumors . Similarly, inhibitors of CSF-1R (to deplete M2 macrophages) and agents targeting adenosine pathways (to prevent T cell suppression) are under development. Even radiation and certain chemotherapies, when given in low doses (metronomic dosing), act as TME modulators by normalizing vessels and stimulating immune surveillance. Furthermore, novel approaches like oncolytic viruses directly infect tumor cells and spark inflammation in the TME, helping to turn “cold” tumors “hot.” These diverse applications demonstrate the real-world use of microenvironment remodeling – often as combination regimens – to break down the tumor’s protective barriers and sensitize it to therapy.
- Advantages Remodeling the tumor microenvironment provides strategic benefits that conventional therapies alone often cannot achieve. One major advantage is the reduction of therapy resistance: by targeting the supportive cells and factors on which tumors rely, TME-directed treatments can prevent or overcome mechanisms that make cancer cells unresponsive to chemo or immunotherapy. Notably, unlike cancer cells which mutate rapidly and develop drug resistance, the non-cancerous cells in the TME are genetically more stable, making them less likely to acquire resistance mutations . This means therapies against the TME (such as anti-VEGF or anti-TGF-β agents) can remain effective longer and hit the tumor in a “soft spot” that doesn’t evolve as quickly as the cancer itself . Another advantage is improved efficacy of other treatments: remodeling the TME often complements and boosts standard therapies. For example, normalizing blood vessels alleviates hypoxia and high interstitial pressure, which allows deeper penetration of drugs and infiltration of immune cells; as a result, previously resistant tumors can become vulnerable to chemotherapy, radiation, or immune attack . By mitigating immunosuppression (e.g. depleting Tregs and MDSCs), TME therapies can amplify the patient’s own anti-tumor immunity, leading to more robust and durable responses than with conventional treatment alone. Importantly, TME-targeted approaches can be quite selective for the tumor site – they aim at features of the tumor’s environment (like abnormal vessels or activated fibroblasts) that are distinct from normal tissue, potentially resulting in fewer side effects when properly targeted. In summary, tumor microenvironment remodeling attacks cancer indirectly but effectively: it disables the tumor’s support system and defensive shield, thereby enhancing the impact of direct tumor-killing therapies and offering patients a better chance at lasting control over their disease .
What medical services does AllCancer provide?
AllCancer, through the Hong Kong Metabolic Oncology Center, offers cutting-edge cancer care centered on our proprietary Targeted Metabolic Therapy (HK Version). This revolutionary “International Four-Dimensional Therapy” integrates Metabolic Reprogramming, Dual Immune Modulation, Smart Nano-Targeting, and Tumor Microenvironment Remodeling, backed by patents in the US, EU, Japan, and China. Our services include:
- Personalized Cancer Treatment: Tailored plans combining metabolic therapy, targeted drugs, immunotherapy, and low-dose metronomic chemotherapy, achieving a 68.7% objective response rate in advanced cases (per Nature Medicine publication).
- Advanced Diagnostics: State-of-the-art imaging (e.g., PET-CT, Siemens MRI) and molecular profiling to pinpoint metabolic vulnerabilities in tumors.
- Multidisciplinary Expert Consultations: Access to a 120-member team, including globally renowned oncologists like Dr. Li Guohua and Prof. Liu Guolong, with seamless coordination across 17 countries.
- Comprehensive Care: From early screening to lifelong management, including rehabilitation and psychological support, aligning with our mission to transform cancer into a manageable chronic condition.
- Clinical Trials: Participation in global multi-center RCTs (e.g., NCT04820250113) for innovative therapies. Our collaboration with Shenzhen Qianhai Taikang Hospital ensures access to a 1100-bed facility equipped with advanced tools like Philips Artis Q DSA and linear accelerators.
What cancers are treated at AllCancer?
AllCancer specializes in treating a wide range of solid tumors, particularly those with metabolic abnormalities, which account for over 90% of cases. Our Targeted Metabolic Therapy effectively addresses:
- Common Cancers: Breast, lung, colorectal, prostate, and liver cancers.
- Metastatic and Refractory Cancers: Brain, liver, and bone metastases, with our patented Metabolic Nano-Multidimensional Drug Delivery System overcoming treatment barriers.
- Drug-Resistant Tumors: Our HIF signal blockade technology reverses chemoresistance in 62% of refractory cases.
- Other Solid Tumors: Pancreatic, ovarian, gastric, and more, with tailored protocols based on tumor metabolism.
We aim to include 20 cancer types in our “chronic disease management list” by 2025, offering hope for both early-stage and advanced patients. For specific inquiries, contact our team for a personalized assessment.
How can I make an appointment with the AllCancer medical team?
Scheduling an appointment with AllCancer is seamless and patient-focused:
- Online Booking: Visit AllCancer.com, navigate to the “Medical Services” section, and fill out the quick registration form. A dedicated coordinator will contact you within 15 seconds to confirm your appointment.
- Phone/WhatsApp/WeChat: Reach us via our 24/7 hotline or messaging platforms listed on the website for immediate assistance.
- Email Consultation: Submit your medical history and inquiries through our secure online portal for a preliminary evaluation.
- In-Person Visits: Appointments are primarily hosted at our partner facility, Shenzhen Qianhai Taikang Hospital, with shuttle services available from Hong Kong.
Book now to secure a consultation with our world-class experts.
What should I bring to my first appointment?
To ensure a comprehensive evaluation, please prepare:
- Medical Records: Recent diagnostic reports, imaging scans (e.g., CT, MRI, PET-CT), pathology results, and treatment history. Digital or physical copies are accepted.
- Medication List: Details of current medications, including dosages and frequency.
- Identification: Valid ID or passport for registration.
- Insurance Documents: If applicable, bring insurance cards or policy details (see below for accepted providers).
- Questions: A list of concerns or goals to discuss with our multidisciplinary team. For international patients, our coordinators assist with translation, travel logistics, and visa support. Upload records via AllCancer.com’s secure portal before your visit to expedite the process. Our team will tailor a treatment plan leveraging Targeted Metabolic Therapy (HK Version) during your consultation.
What insurance companies does AllCancer accept?
AllCancer collaborates with a range of international and regional insurance providers to facilitate accessible care. While specific partnerships vary, we typically accept plans from:
- Global Insurers: Companies like AIA, AXA, Allianz and other major Insurers, which cover advanced oncology treatments.
- Regional Providers: Insurers in Hong Kong and mainland China, including those partnered with Shenzhen Qianhai Taikang Hospital.
- Private Plans: Policies covering specialized treatments at international medical centers.
To confirm coverage, submit your insurance details through AllCancer.com’s Medical Office or contact our billing team via WhatsApp/WeChat. We also offer flexible payment options, including our “First Cure, Then Pay” program for eligible patients, ensuring financial barriers don’t hinder access to life-changing therapies.