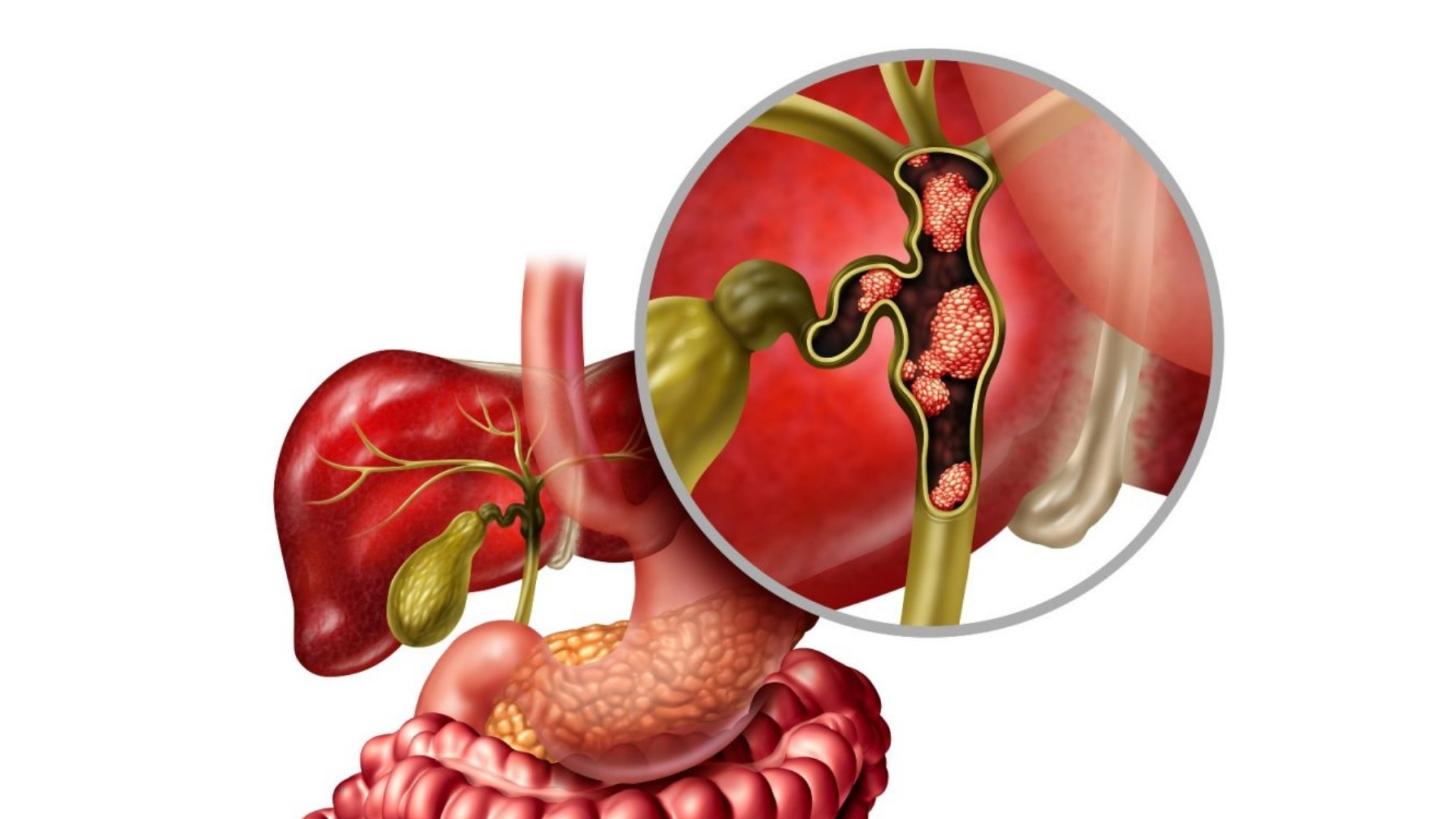
What is Bile Duct Cancer (Cholangiocarcinoma)?
Bile Duct Cancer (Cholangiocarcinoma) is an aggressive form of cancer arising from epithelial cells lining the bile ducts. Bile ducts serve as drainage channels for bile—a fluid essential for digestion produced by the liver. When these cells become malignant, they multiply uncontrollably, impairing bile flow. Understanding Bile Duct Cancer (Cholangiocarcinoma) helps empower patients, fostering hope through informed treatment decisions and awareness.
Cholangiocarcinoma is traditionally classified according to the anatomical location affected, including:
- Intrahepatic Cholangiocarcinoma (iCCA): Occurs inside liver parenchyma.
- Perihilar Cholangiocarcinoma (Klatskin Tumors): Develops at junction of left and right hepatic ducts.
- Distal Cholangiocarcinoma: Found within the bile ducts near small intestine.
At the cellular level, cholangiocarcinoma demonstrates hallmark metabolic vulnerabilities, like heightened reliance on glucose, known as the Warburg effect, where cancerous cells consume glucose up to 200 times faster than normal cells. Targeting these metabolic characteristics offers promising new therapeutic interventions, currently being pioneered extensively at AllCancer.
The incidence of Cholangiocarcinoma has seen a marked increase in recent years, particularly in Asia. Globally, an estimated 165,000 new cases are diagnosed annually according to WHO 2024 data. Particularly prevalent in Southeast Asian regions, including Thailand, South Korea, Japan, and notably Hong Kong, cholangiocarcinoma exhibits a higher frequency attributable in part to chronic liver diseases such as viral hepatitis (Hepatitis B in particular), liver flukes infestation, and other local epidemiological factors.
In Hong Kong specifically, cholangiocarcinoma affects roughly 8–12 in 100,000 individuals yearly, with rising cases predicted due to demographic shifts driving prolonged life expectancies and aging populations. Age significantly influences cholangiocarcinoma risk, typically affecting adults in their 60s–70s, though younger cases have emerged. Males have been slightly more prone than females, possibly reflecting occupational and lifestyle differentials.
Patients newly diagnosed typically face considerable emotional and physical impacts, experiencing devastating symptoms including:
- Jaundice: Yellowing skin, eyes due to bilirubin accumulation.
- Chronic fatigue: Exhaustion due to impaired liver function.
- Abdominal pain and bloating: Resulting from tumors obstructing bile flow.
- Poor digestion and appetite loss, leading to rapid weight reduction.
- Psychological burdens: Anxiety, depression, and fear accompanying cancer diagnosis.
Addressing these impacts compassionately through supportive care options and revolutionary treatments, AllCancer integrates patient-oriented approaches, helping individuals regain a sense of control, optimism, and enhancing overall well-being.
Moreover, leveraging Nobel Prize-guided metabolic therapies (inspired by laureates Dr. Jim Allison and Dr. Gregg Semenza), AllCancer offers innovative approaches to exploit cancer’s metabolic vulnerabilities specifically targeting Cholangiocarcinoma. Metabolically targeting the abnormal glucose-dependent behavior of tumor cells allows treatments such as the specialized 4D Metabolic Therapy, significantly improving patient outlooks.
Bile Duct Cancer (Cholangiocarcinoma) Overview in Hong Kong
Integrating regional healthcare insights, AllCancer collaborates extensively with acclaimed Hong Kong oncologists including Dr. Li Guohua and Prof. Liu Guolong, notable pioneers in Metabolic Oncology, whose authoritative research has garnered recognition in Nature Medicine and secured international patents across the US, EU, Japan, and China. This scientific excellence reassures patients, trustingly endorsing revolutionary treatments customized to local epidemiology.
Embracing Hope and Leveraging Innovations
Crucially, believing every patient deserves focused, compassionate care, AllCancer advances groundbreaking clinical programs integrating compassionate initiatives including the remarkable “Cure First, Pay Later” policy in cooperation with Shenzhen Qianhai Taikang and globally acclaimed institutions such as MD Anderson Center. Embracing such progressive measures enlightens every patient’s journey—furthering AllCancer’s ambitious 2025 aim to manage cholangiocarcinoma as a chronic, treatable disease.
Causes and Risk Factors of Bile Duct Cancer (Cholangiocarcinoma)
Understanding Bile Duct Cancer (Cholangiocarcinoma) risk factors empowers proactive measures, driving earlier diagnosis and optimized outcomes. Cholangiocarcinoma arises from several intertwined genetic, environmental, lifestyle, and metabolic dynamics.
Genetic Risk Factors for Cholangiocarcinoma
- BRCA mutations: Genetic predispositions, specifically BRCA1/2 gene mutations involved in DNA repair, can sometimes increase cholangiocarcinoma susceptibility.
- EGFR and KRAS mutations: Specific mutations accelerating cell growth and division have been detected increasingly in cholangiocarcinoma cases, suggesting valuable diagnostic markers.
Environmental and Lifestyle Factors Impacting Risks
- Chronic Hepatitis Infection: Particularly Hepatitis B and C, highly prevalent in Asia, has been directly correlated with increased cholangiocarcinoma incidence, specifically in Hong Kong’s aging populace.
- Liver Fluke Infestation: Endemic in SEA regions, this parasitic infection directly links to cholangiocarcinoma risk.
- Exposure to Industrial Chemicals: Frequent occupational exposures (rubber, textile, chemical industries) potentially escalate risks.
Metabolic Vulnerabilities: A Therapeutic Opportunity
AllCancer highlights targetable metabolic vulnerabilities especially glucose-dependency (Warburg effect) and glutamine-dependency critical in nucleotide biosynthesis pathways, opening avenues for novel therapies including AllCancer’s proprietary “4D Metabolic Therapy”—a groundbreaking innovation dramatically reshaping cholangiocarcinoma treatment paradigms.
Taking action through early intervention and expert diagnostics becomes vital. Explore promising solutions today—Discover how AllCancer’s pioneering 4D Therapy transforms cholangiocarcinoma management prospects.
Symptoms of Bile Duct Cancer (Cholangiocarcinoma)
Early recognition of symptoms is crucial for improving outcomes in Bile Duct Cancer (Cholangiocarcinoma). Symptoms typically vary by stage and can be subtle initially. Patients are encouraged to promptly consult medical professionals upon experiencing any notable signs.
Early Signs of Bile Duct Cancer (Cholangiocarcinoma)
- Jaundice (yellowing of the skin and eyes)
- Pale-colored stools
- Dark-colored urine
- Generalized itching (pruritus)
- Unexplained weight loss
- Abdominal pain or discomfort (typically upper right quadrant)
- Loss of appetite or feeling of fullness after minimal eating
- Fatigue and lethargy
Notably, jaundice is one of the most significant early indicators of Bile Duct Cancer (Cholangiocarcinoma). This occurs due to obstruction in bile flow as a result of tumor growth. The tumor biology reflects obstruction at the bile duct or surrounding structures, impairing normal bile drainage and liver function.
Advanced Stage Symptoms of Bile Duct Cancer (Cholangiocarcinoma)
- Persistent fatigue
- Severe abdominal pain
- Fever and infection due to bile duct inflammation (cholangitis)
- Significant, ongoing weight loss (cachexia)
- Swelling of the abdomen (ascites)
- Nausea and vomiting
- Digestive issues and malnutrition due to impaired digestion of dietary fats
Advanced-stage Cholangiocarcinoma indicates extensive tumor invasion or metastatic spread, affecting liver function and possibly neighboring organs such as the pancreas or gallbladder. Early consultation and diagnostic evaluation substantially increase treatment options, improving survival rates and quality of life.
Why Early Detection Matters
Identifying symptoms at the outset reduces the likelihood of complicated treatments and improves quality of life. Patients experiencing early signs such as jaundice or unexplained digestive issues are strongly recommended to seek immediate medical advice for effective early intervention.
Stages of Bile Duct Cancer (Cholangiocarcinoma) and Survival Rates
Stage 1 – Bile Duct Cancer (Cholangiocarcinoma)
Stage 1 bile duct cancer refers to a localized tumor, confined strictly within the bile ducts, without any lymph node involvement.
- Tumor typically small and fully localized
- Possibility for complete surgical resection is high
- 5-year survival rate: approximately 80% if fully resectable (Hong Kong Cancer Registry 2024)
- Preferred treatments: Surgical resection, targeted radiotherapy, adjuvant chemotherapy
At this stage, complete removal of the tumor is often feasible and can substantially improve survival prospects and patient outcomes.
Stage 2 – Bile Duct Cancer (Cholangiocarcinoma)
Stage 2 involves larger tumors or early evidence of invasion outside the bile ducts or nearby soft tissues. Regional lymph node involvement may or may not be observed.
- Larger tumor size compared to stage 1, possible lymph node involvement
- Recommended treatments: Surgical resection with postoperative radiotherapy, chemotherapy
- 5-year survival rate: approximately 50-60% (data based on Asian populations reported by Hong Kong Oncology Society guidelines)
Multimodal treatments often result in improved prognostic outcomes at this stage, with earlier intervention strongly advocated.
Stage 3 – Bile Duct Cancer (Cholangiocarcinoma)
Stage 3 Cholangiocarcinoma demonstrates extensive regional involvement, including proliferation into or beyond regional lymph nodes, nearby structures, or adjacent organs.
- Extensive regional lymphatic spread and tumor growth
- Recommended treatments: Combined surgery and chemotherapy; targeted and radiation therapies in select cases
- 5-year survival rate: roughly between 20-40% in Asian cohorts, reflecting studies by the Asian-Pacific Hepato-Pancreato-Biliary Association (2024)
Given significant tumor burden at stage 3, an integrative and personalized approach improves clinical outcomes and may prolong patient survival.
Stage 4 – Bile Duct Cancer (Cholangiocarcinoma)
In stage 4, the cancer has metastasized to distant organs such as the lungs or liver, significantly complicating treatment.
- Metastatic spread detected in distant anatomical regions
- Favored treatments: chemotherapy, targeted metabolic therapies (capitalizing on cancer cells’ reliance on glucose metabolism and glutamine dependency), immunotherapy options
- 3-year survival rate: approximately 10-20% (data from Hong Kong Cancer Registry and Asia-Pacific studies, 2025)
- Increasing interest in novel therapies aiming for chronic disease management, consistent with progressive strategies targeted by experts such as Dr. Li Guohua and Prof. Liu Guolong
Despite the stage 4 severity, innovative approaches and ongoing research endeavours in metabolic and targeted therapies aim to improve chronic management outcomes, aligning with AllCancer’s mission to transform cancer into manageable chronic conditions.
Patients encountering any stage-specific symptoms or requiring further information on treatment options, particularly novel metabolic therapies like AllCancer’s cutting-edge “4D Therapy,” are urged to explore further medical consultation.
Treatment Options for Bile Duct Cancer (Cholangiocarcinoma)
Standard Surgical Treatments
Surgical interventions remain the cornerstone of curative treatment for bile duct cancer (Cholangiocarcinoma), particularly when diagnosed in early stages. Procedures include:
- Bile Duct Resection: Removal of affected bile duct segments, with reconnection to restore bile flow.
- Partial Hepatectomy: Liver resection often combined when cancer has invaded hepatic tissues, crucial for intrahepatic cholangiocarcinoma.
- Whipple Procedure (Pancreatoduodenectomy): Extensive surgery for distal cholangiocarcinoma, removing head of pancreas, duodenum, gallbladder, and bile duct segments.
In Hong Kong and Asia, surgical management often demands expert multidisciplinary teams highly specialized in advanced hepatic and biliopancreatic procedures. Early-stage surgical treatments provide significant survival benefits, potentially offering patients prolonged remission or complete recovery when cases are localised.
Chemotherapy and Radiation Therapy
Chemotherapy continues to be a primary option when surgical removal isn’t achievable or when the disease has spread significantly. For cholangiocarcinoma, commonly used chemotherapy regimens include:
- Cisplatin and Gemcitabine: A standard regimen known to improve survival and quality of life.
- Oxaliplatin-based therapies: Often selected when cisplatin is unsuitable due to toxicity or intolerances.
- Capecitabine: Used either as monotherapy or in combination, specifically post-surgery for adjuvant treatment.
Radiation therapy, either external beam radiation or intensity-modulated radiation therapy (IMRT), may be employed to reduce tumor size, alleviate symptoms, and prevent recurrence postoperatively. In Asia, due to limited treatment infrastructure in certain regions, radiation therapy access can sometimes be challenged by patient logistics and resource availability.
Targeted and Immunotherapy Treatments
Recent advancements have led to innovative targeted therapies aimed specifically at the cancer cells’ biological vulnerabilities. Key targeted treatments currently investigated or deployed in clinical practice for cholangiocarcinoma treatments include:
- FGFR inhibitors (e.g., Pemigatinib, Infigratinib): Showing promise in cholangiocarcinoma with FGFR2 fusions or rearrangements.
- IDH inhibitors (e.g., Ivosidenib): Effective in tumors harboring IDH1 mutations, reducing tumor growth and potential metastasis effectively.
- Immune checkpoint inhibitors (e.g., Pembrolizumab): Enhancing the immune system’s capability to identify and attack cancer cells particularly for MSI-high cholangiocarcinoma.
Despite promising potential, patients in Asia often have limited access to novel therapies due to regulatory delays and constrained healthcare resource allocation, emphasizing a strong need to accelerate the integration of innovative drug platforms across the region.
Emerging Metabolic Therapies
Understanding cancer cell metabolism, especially given the Warburg effect, is expected to revolutionize cholangiocarcinoma therapy. Emerging metabolic therapies focus on exploiting cancer cells’ high dependency on glucose and glutamine for survival and proliferation:
- Blocking GLUT1/3 Glucose Transporters: Cutting cellular nutrient uptake cripples cholangiocarcinoma cells’ excessive glucose consumption, significantly impeding tumor growth.
- Glutamine pathway inhibitors: Preventing glutamine metabolism weakens tumoral energy production, making cancer cells highly susceptible to routine therapies.
In Asia-Pacific and Hong Kong regions, AllCancer’s metabolic oncology center pioneers these advanced therapies, aiming to improve outcomes and quality of life, especially in metabolically resistant cholangiocarcinoma cases.
Limitations of Traditional Therapies for Bile Duct Cancer (Cholangiocarcinoma)
Chemotherapy Limitations
While chemotherapy remains vital in cholangiocarcinoma management, numerous challenges limit its effectiveness:
- Significant systemic toxicity causing serious bone marrow suppression occurs frequently, with estimates suggesting nearly 78% experiencing hematological side effects.
- Cardiac toxicity reported in approximately 23% of cases, highlighting particular concerns during prolonged treatments.
- Severe gastrointestinal disturbances such as nausea, vomiting, and loss of appetite constrict patient tolerance and impair treatment adherence.
Particularly in Asia and Hong Kong, limited patient counseling, logistical resources, and access barriers further complicate chemotherapy application and decrease treatment adherence, compromising patient survival outcomes.
Radiation Therapy Drawbacks
Radiation therapy too introduces complexities that sometimes outweigh therapeutic benefits:
- High incidents of adjacent tissue damage often resulting in digestive system issues like inflammatory colitis or non-cancerous hepatic injury, further debilitating patients undergoing treatment.
- Increased risk for secondary malignancies especially prominent after extensive radiation treatment courses, raising secondary cancer risk by up to 300% according to recent JAMA Oncology reports.
In resource-limited Asia-Pacific healthcare settings, comprehensive monitoring and sophisticated radiotherapy is subdued, presenting considerable operational hurdles in effectively deploying safe radiotherapy interventions.
Surgical Risks and Limitations
Surgical interventions, albeit potentially curative, carry significant perioperative risks:
- Risk of severe infection and postoperative complications, particularly high (15-25%) in extensive resections like Whipple procedures.
- Prolonged hospitalization and high healthcare costs due to complications and extended recovery.
- Limited applicability to less than 30% patients due to late detection and advanced tumor stages during diagnosis, significantly limiting surgery’s role in cholangiocarcinoma.
Considering late-stage identification tied to limited early diagnostic strategies in Hong Kong and broader Asia, surgical application is notably constrained, necessitating heightened awareness campaigns for earlier patient identification and intervention.
Metabolic Resistance Mechanisms
Cancer cells develop intrinsic metabolic adaptability to reduce therapy effectiveness such as:
- Remarkably increased DNA repair enzyme activity (upward of 400%) that counteracts chemotherapy-driven DNA damage.
- Altered nutrient utilization and energy metabolisms such as heightened glycolysis, providing a significant resistance pathway against conventional cytotoxic strategies.
These resistance mechanisms highlight the limitations in Asia’s limited approach to traditional therapies, emphasizing the critical necessity towards integrating emerging targeted and metabolic strategies into routine clinical practice across the region.