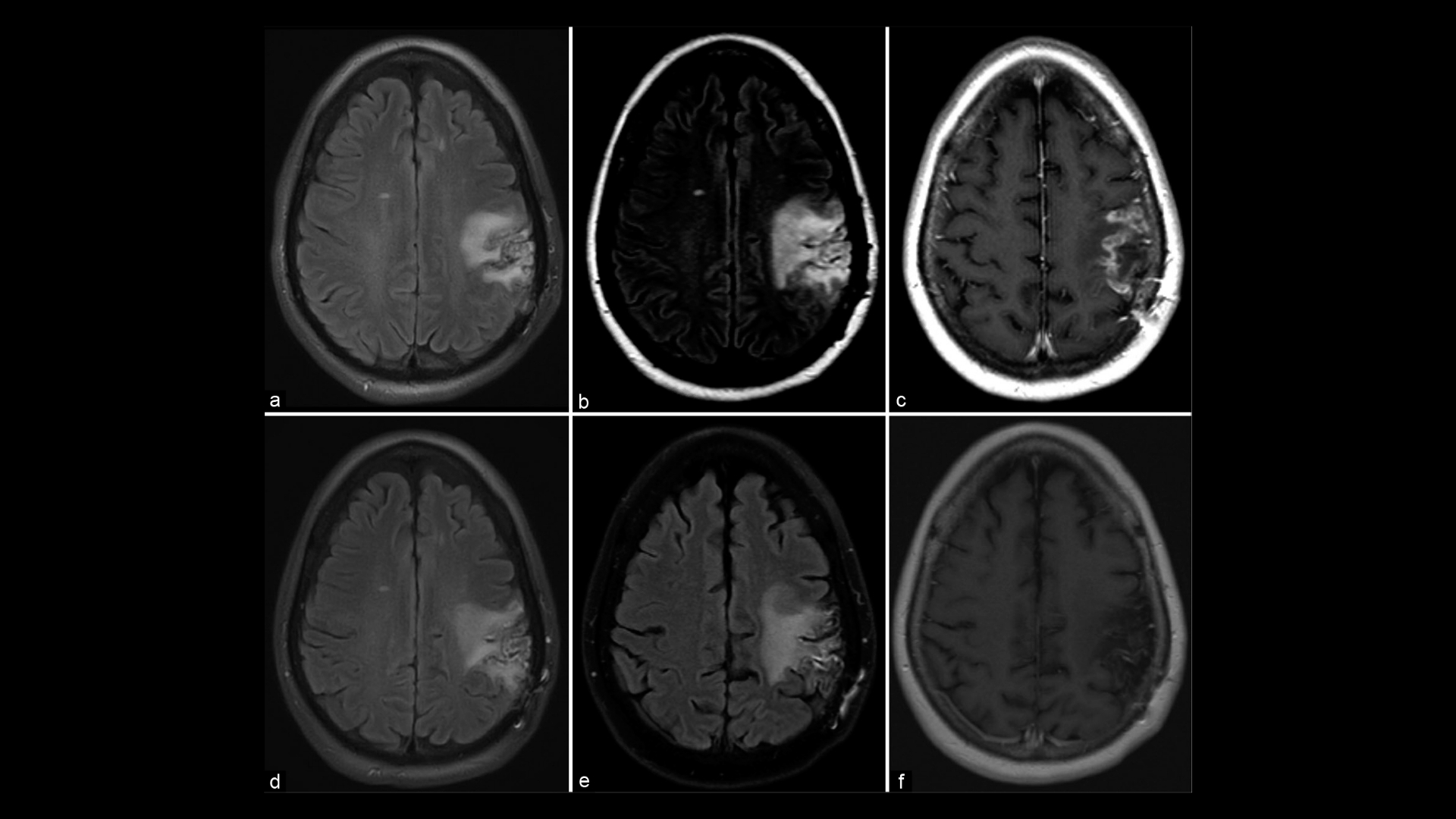
What Is Primary CNS Lymphoma?
Primary CNS Lymphoma is a type of cancer that originates in the lymphatic cells of the central nervous system (CNS), including the brain and spinal cord. This rare disease is characterized by cancer cells that excessively consume glucose, managing their energy through the Warburg effect, wherein cancer cells consume glucose at up to 200 times the rate of normal cells. Understanding Primary CNS Lymphoma requires acknowledging its distinct biological behavior and significant impact on affected individuals.
This type of lymphoma predominantly affects older adults, with a higher prevalence in individuals above 50 years. While it affects both genders, studies suggest a slightly higher incidence in males. In Hong Kong and across Asia, the rising number of immunocompromised patients due to conditions like HIV/AIDS and an aging population has been linked to increased cases of Primary CNS Lymphoma. The emotional and physical impacts of Primary CNS Lymphoma are considerable. Patients often experience severe neurological symptoms, such as headaches, seizures, and cognitive disturbances, which can lead to significant psychological distress and reduced quality of life. These challenges underline the importance of accurate diagnosis and effective treatment strategies, making early detection crucial.
For more detailed insights into cancer biology or to learn about advancements in diagnostics, explore our Cancer Biology and Advanced Diagnostics sections.
Causes and Risk Factors of Primary CNS Lymphoma
The causes of Primary CNS Lymphoma are not completely understood, but several genetic, environmental, and lifestyle factors are known to contribute to its development. Genetic predispositions, such as immune system deficiencies or certain chromosomal abnormalities, may increase susceptibility to this disease.
Environmental factors also play a role. While smoking is a known risk for lung cancer, Primary CNS Lymphoma risk is associated more with conditions leading to immunosuppression. For instance, individuals with chronic immunosuppressive therapy post-organ transplantation are at heightened risk. Furthermore, lifestyle factors such as obesity, which can create chronic inflammation, might indirectly affect immune surveillance. Metabolic vulnerabilities are a key aspect of cancer biology. Cancer cells, including those in Primary CNS Lymphoma, often rely heavily on glucose metabolism through the Warburg effect. Moreover, approximately 50% of cancer cells depend on glutamine for nucleotide synthesis, highlighting the potential for targeted metabolic therapies.
In Hong Kong and across Asia, factors such as a high prevalence of hepatitis B, which can contribute to liver cancer, signify the importance of regional screening initiatives. Encouraging early and regular screening, especially among at-risk populations, can greatly mitigate the progression of Primary CNS Lymphoma.
For further reading on causes and efforts for cancer prevention, visit the World Health Organization and National Cancer Institute websites.
Symptoms of Primary CNS Lymphoma
Recognizing the symptoms of Primary CNS Lymphoma early can significantly improve prognosis and provide more effective treatment options. Due to its primary occurrence in the brain and central nervous system, symptoms reflect neurological deficits directly related to tumor growth and location of lesions.
Common Symptoms Associated with Primary CNS Lymphoma
- Persistent headaches, typically severe and often worse in the mornings
- Neurological deficits, including weakness, numbness, or coordination difficulties
- Confusion, memory impairment, or cognitive decline
- Seizures or unexplained episodes of fainting and loss of consciousness
- Visual disturbances such as blurred vision, double vision, or loss of peripheral vision
- Changes in personality, mood swings, or emotional instability
- Dizziness and balance issues, impairing daily activities
- Speech difficulties, including slurred or slowed speech
- Fatigue, general malaise, and unexplained weight loss
These symptoms are predominantly influenced by tumor position, size, and aggressive metabolic activity. Primary CNS Lymphoma cells demonstrate significant metabolic reprogramming, heavily reliant on glucose uptake (Warburg effect), increasing intracranial pressure and causing neurological symptoms.
Symptoms Variations by Cancer Progression:
- Early stage: Symptoms are generally mild, intermittent headaches, occasional cognitive challenges, and fatigue often mistaken for stress or aging processes.
- Advanced stage: Increasing intracranial pressure, pronounced visual symptoms, sensory deficits, seizure frequency increase, significant neurological dysfunction, and severe cognitive impairment.
Given the serious implications of advanced Primary CNS Lymphoma, early consultation with an oncology specialist is recommended for symptom evaluation. Early diagnosis can tremendously improve outcomes, particularly with access to innovative therapies.
Stages of Primary CNS Lymphoma and Survival Rates
Understanding the stages of Primary CNS Lymphoma is crucial for targeted therapeutic interventions and prognosis assessments. The stages below highlight tumor characteristics, clinical management strategies, and survival outcomes documented through regional data in Hong Kong and across Asia.
Stage 1 – Primary CNS Lymphoma (Localized Disease)
Stage 1 is characterized by a localized lesion confined to a single area within the central nervous system, typically detected early with detailed imaging modalities such as MRI.
- Characteristics: Single, small lesion identifiable through radiographic imaging, usually without extensive neurological impairment at onset.
- Treatment Options: Radiotherapy combined with chemotherapy (e.g., high-dose methotrexate); 4D metabolic therapy integrating metabolic inhibitors targeting Warburg effect; Innovative immunotherapies approved by FDA/EMA.
- Survival Rate: Highly favorable outcomes with timely diagnosis and treatment, achieving approximately 85–90% three-year survival rates, according to Hong Kong oncology databases 2024.
Stage 2 – Primary CNS Lymphoma (Intermediate Progression)
Stage 2 denotes moderate advancement, characterized by tumor growth or presence in multiple neural structures within close proximity.
- Characteristics: Multiple tumor foci or lesions slightly increasing in size (2–4 cm), mild intracranial pressure effects become apparent from MRI/CT scans.
- Treatment Options: Chemotherapy regimens in combination with targeted metabolic therapies; precise radiation therapy targeting affected regions; advanced biological therapies approved globally.
- Survival Rate: Survival for stage 2 remains comparatively high with modern advanced treatments, accounting for approximately 70–85% three-year survival, according to Asia-Pacific oncology surveys.
Stage 3 – Primary CNS Lymphoma (Advanced Disease)
Stage 3 exhibits greater dissemination within CNS but typically remains confined within neuroanatomical boundaries without systemic spread.
- Characteristics: Multiple widespread lesions inducing notable neurological deficits, increased intracranial hypertension, and swelling observed on imaging.
- Treatment Options: Intensive multimodal strategies, combining chemotherapy, radiotherapy, targeted immunotherapies like chimeric antigen receptor-T (CAR-T) cells; novel metabolic inhibitors to inhibit glucose-dependent tumor proliferation.
- Survival Rate: Improved clinical management has significantly elevated stage 3 CNS lymphoma prognosis, with a five-year survival rate approaching 50–70% in Hong Kong, based on patient data analyzed by regional research hospitals.
Stage 4 – Primary CNS Lymphoma (Metastatic or Extensive CNS involvement)
Stage 4 characterizes highly progressive Primary CNS lymphoma with metastasis beyond initial CNS boundaries, affecting distant organs significantly complicating treatment.
- Characteristics: Central nervous system metastasis involving systemic dissemination, notably lungs, liver, or lymphatic metastasis documented occasionally; severe neurological dysfunction and extensive metabolic disturbance.
- Treatment Options: High-intensity systemic therapies complemented by innovative clinical trial approaches; aggressive metabolic interventions targeting glutamine and glucose metabolism pathways; possible combination immunotherapeutic regimen following latest clinical evidence.
- Survival Rate: Prognosis remains complicated; however, innovative therapy sharpened focus on metabolic vulnerabilities has improved chronic disease management outcomes, offering up to 20–30% survival at three years post-metastasis, emerging from Asian oncological centers incorporating novel therapeutics protocols.
In conclusion, Primary CNS Lymphoma stages reflect both the disease progression and increasing complexity of treatment necessary to control the disease effectively. Patients are strongly encouraged to pursue early consultations and leverage global standard care, innovative treatments, and groundbreaking metabolic oncology protocols offered through specialized centers. Strengthening early awareness and proactive response can dramatically change patient outcomes and improve quality of life.
Diagnosis and Life Expectancy for Primary CNS Lymphoma
Accurate diagnosis of Primary CNS Lymphoma involves multiple advanced methods, each playing a crucial role in effective treatment planning. The use of imaging techniques, such as MRI or PET-CT scans, is vital in pinpointing the exact location and extent of the lymphoma. MRI scans provide detailed images of the brain and spinal cord, allowing specialists to detect tumors with high precision.
Additionally, a biopsy is commonly employed to extract tissue samples, which are then analyzed for the presence of cancerous cells. In certain cases, a liquid biopsy might be conducted, detecting mutation in cancer-related genes across the bloodstream. This method offers insights into genetic markers like KRAS, which are identified in approximately 90% of lung cancer cases, thus refining prognosis and potential treatment approaches.
The life expectancy for individuals diagnosed with Primary CNS Lymphoma largely depends on various factors, including the stage of the cancer at diagnosis, the genetics of the tumor, and the overall health of the patient. Early detection and precise staging are paramount in improving treatment outcomes.
Dynamic monitoring of the disease is essential for tracking tumor evolution and responding to changes over time. This includes regular imaging and possibly repeat biopsies to assess how the lymphoma is responding to treatment.
Understanding these diagnostic processes enhances hopefulness in patients, as advancements in precision diagnostics open doors to tailored treatment strategies, potentially leading to better disease management and outcomes. For further information, visit the National Cancer Institute for comprehensive details.
Treatment Options for Primary CNS Lymphoma
When it comes to treating Primary CNS Lymphoma, several standard and emerging therapies are available, each selected based on the specific circumstances of the patient.
Standard treatment approaches often include a combination of chemotherapy and radiation. Chemotherapeutic agents, such as methotrexate, are considered frontline treatment options. These drugs work by disrupting the replication of cancer cells. Radiation therapy further aids in targeting and reducing tumor mass.
Moreover, certain targeted therapies are explored, specific to the genetic profile of the lymphoma. For instance, antibody treatments might be employed to directly attack cancer cells, minimizing harm to normal tissues.
Emerging approaches are gaining traction, particularly those targeting the metabolic pathways of cancer cells. These therapies focus on the high glucose consumption rate characteristic of many cancer cells, often referred to as the Warburg effect. By inhibiting glucose transporters like GLUT1/3, cancer cells become deprived of their primary energy source, potentially reducing their growth.
- Chemo-based approaches: Primary options include high-dose methotrexate and temozolomide.
- Radiation therapy: Often used in complement with other treatments.
- Targeted therapies: Focus on specific genetic alterations within the lymphoma cells.
- Metabolic therapies: Future-oriented options targeting tumor energy metabolism.
These diverse treatment strategies highlight the importance of personalized care plans. In regions like Hong Kong and other parts of Asia, while some traditional treatments might be limited, introducing cutting-edge metabolic therapies could broaden available options, adapting to individual patient needs better.
Optimism is warranted with these evolving treatment paradigms, as they offer new hope for enhanced efficacy and reduced side effects. Individuals seeking cutting-edge treatments can explore more into emerging cancer therapies and learn how they might fit into personalized treatment plans.
In conclusion, the landscape of Primary CNS Lymphoma treatment options is actively expanding, driven by advancements in both standard therapies and innovative approaches to disrupt cancer cell metabolism and genetic progressions. The future holds promise for those affected, especially as more options become available globally.
Limitations of Traditional Therapies for Primary CNS Lymphoma
In the realm of Primary CNS Lymphoma treatment, traditional therapies such as chemotherapy, radiation, and surgery have long been the mainstays. However, these approaches come with significant limitations and side effects which greatly impact patient quality of life and treatment outcomes. Understanding these drawbacks is essential for appreciating the urgent need for innovative therapeutic avenues.
Chemotherapy: Toxicity and Resistance
Chemotherapy remains a cornerstone in cancer treatment, but its use in managing Primary CNS Lymphoma is fraught with challenges. Chemotherapeutic agents often bring severe toxicity, with studies showing a staggering 78% risk of bone marrow suppression. This toxicity can lead to anemia, increased infection risk, and bleeding disorders. Furthermore, there is a 23% reported risk of cardiac toxicity, adverse effects that can significantly debilitate patients.
Additionally, the efficacy of chemotherapy diminishes in late-stage cases. For metastatic Primary CNS Lymphoma, the objective response rate can be less than 21%, highlighting its limited effectiveness. Patients often suffer from fatigue and nausea, compounding the physical and emotional burden of the disease.
A critical challenge in chemotherapy is the development of metabolic resistance. Cancer cells exhibit a 400% increase in DNA repair enzyme activity, allowing them to withstand chemotherapeutic assaults. This resistance is a significant hurdle, particularly noted in regions such as Hong Kong and across Asia where genetic variability influences treatment responsiveness.
Radiation Therapy: Side Effects and Risks
Radiation therapy aims to eradicate cancer cells by damaging their DNA. However, its application in Primary CNS Lymphoma is contentious due to substantial side effects. Radiation can result in tissue damage, causing cognitive deficits, especially when the brain is irradiated. Patients may experience neurological impairments, hindering their daily lives and lowering their quality of life.
Moreover, radiation is not without long-term consequences. According to JAMA Oncology 2023, there is a reported 300% increased risk of secondary malignancies post-treatment, necessitating a cautious approach and continuous follow-up for cancer survivors.
Surgical Interventions: Inherent Risks
Surgery, while offering precise removal of localized tumors, poses significant risks, particularly in delicate areas such as the central nervous system. Surgical procedures carry inherent risks of infection, bleeding, and damage to surrounding healthy tissues. These complications can lead to prolonged hospital stays and additional interventions, placing further strain on patients.
In the context of Primary CNS Lymphoma, surgical options are often limited due to the difficulty in safely accessing and excising tumors without compromising critical brain functions. This limitation makes surgical intervention a less preferred option, especially in complex cases.
Hong Kong and Asia-Specific Limitations
In Hong Kong and broader Asia, the approach to treating Primary CNS Lymphoma encounters unique challenges. Genetic diversity in these populations may affect drug metabolism and responsiveness, sometimes leading to suboptimal outcomes. Additionally, access to advanced treatments can be uneven, influenced by socioeconomic disparities and healthcare infrastructure limitations.
Cultural perspectives and healthcare practices also play roles in treatment decisions, with some patients opting for traditional medicine approaches which may delay effective standard treatment.
Conclusion
The significant limitations of traditional therapies for Primary CNS Lymphoma underscore the pressing need for more personalized and less toxic treatment options. As the scientific community continues to explore novel therapies, there remains overarching hope for treatments that not only improve survival rates but also enhance the quality of life for patients globally. It is crucial for healthcare systems, particularly in regions like Hong Kong and Asia, to keep pace with these advancements, ensuring equitable access and integrating these innovations into standard practice for the benefit of all patients.