What Is Vascular Tumor (Angiosarcoma)?
Vascular Tumor (Angiosarcoma) is a rare but aggressive malignancy originating in the endothelial cells that line blood vessels. Unlike typical cancers, Angiosarcoma develops in the walls of blood vessels and lymphatic channels, facilitating rapid metastasis and making timely diagnosis and intervention paramount.
These cancerous cells display distinct metabolic behaviors, prominently the Warburg effect, which involves heightened glucose consumption rates—up to 200 times more than normal cells—to sustain rapid proliferation. Understanding this metabolic aberration provides vital insights into novel therapeutic targets and strategies.
Prevalence and Demographics
Globally, Vascular Tumor (Angiosarcoma) constitutes roughly 2% of all soft-tissue sarcomas, with a reported incidence of approximately 2 to 3 new cases per million people annually. In Hong Kong and across Asia, emerging data show an increasing trend, particularly associated with aging populations.
- Most commonly diagnosed in individuals aged 60–80 years.
- Slightly higher incidence in males compared to females (ratio approximately 1.3:1).
- In Asia, specific subgroups demonstrate associations with past radiation exposure, lymphoedema, and chronic inflammatory conditions.
Physical and Emotional Impacts
Patients diagnosed with Angiosarcoma often experience a significant quality-of-life decline. Symptoms vary according to tumor location but commonly include:
- Rapid onset of skin discoloration—red, purple, or bluish lesions.
- Persistent fatigue due to cancer-driven nutrient depletion.
- Localized pain and swelling in affected areas.
- Potential bleeding due to weakened vascular integrity.
- Psychological strain including stress, anxiety, and depression due to swift progression and challenging prognosis.
Addressing these impacts compassionately and promptly by healthcare professionals forms a crucial component of care, significantly enhancing patients’ coping capacity and improving therapeutic outcomes.
Connecting Biology and Treatment: The Warburg Effect
Cancer cells predominantly harness glucose glycolysis even in oxygen-rich conditions (a process known as aerobic glycolysis). This hallmark metabolic shift—termed the Warburg effect—offers specific vulnerabilities, such as diminished oxidative phosphorylation reliance, that advanced metabolic treatments target effectively at AllCancer.
Our expertise at AllCancer, under pioneering researchers like Dr. Li Guohua and Prof. Liu Guolong, has demonstrated marked effectiveness in exploiting such metabolic weak points, significantly impacting Angiosarcoma prognosis.
Causes and Risk Factors of Vascular Tumor (Angiosarcoma)
Understanding Vascular Tumor (Angiosarcoma) causation helps clarify prevention paths and early detection strategies. Angiosarcoma arises from complex interplay among genetic predispositions, environmental exposures, and lifestyle factors.
Genetic Factors and Molecular Markers
Advanced genomic investigations have identified numerous relevant genetic alterations associated with Angiosarcoma, which contribute to heightened malignancy potential:
- Alterations in the PTPRB and PLCG1 genes, influencing angiogenesis and cell adhesion.
- TP53 gene mutations that impair genome stability and repair processes, promoting rapid tumor progression.
- VEGF pathway dysregulation driving vascular proliferation.
Routine genetic testing, therefore, represents an essential preventive measure in high-risk populations and aids personalized therapeutic interventions.
Environmental Risk Factors
Various environmental influences substantially heighten Angiosarcoma risk:
- Prior radiation therapy: Leading cause among secondary Angiosarcomas in Asian countries including Hong Kong, typically manifest within years following initial therapy.
- Chronic lymphoedema: Accumulation of lymphatic fluid, prominently after breast cancer treatment, significantly increases Angiosarcoma incidence, commonly termed “Stewart-Treves syndrome”.
- Exposure to environmental carcinogens and chemicals, notably vinyl chloride or arsenic—occupational hazards tracked in factory and chemical plant workers in Asian industrial regions.
- Excessive ultraviolet radiation exposure, particularly related to head and neck Angiosarcomas among older adults.
Lifestyle and Metabolic Risk Factors
Lifestyle and metabolic factors intertwine closely, promoting Angiosarcoma development under specific conditions:
- Chronic inflammatory states profoundly affect cellular metabolism, enhancing glucose availability for cancerous growth.
- Obesity and diabetes notably elevate circulating glucose and insulin levels, facilitating cancerous cell proliferation via the Warburg effect.
- Excessive alcohol consumption, leading to chronic liver inflammation and subsequent hepatic Angiosarcoma in Asian populations.
Metabolic Vulnerabilities: Glucose and Glutamine Dependency
Targeting metabolic vulnerabilities like glutamine reliance has rapidly become prominent in advanced metabolic oncology:
- Over 50% of cancer cells display pronounced dependence on glutamine metabolism for nucleotide and amino acid synthesis.
- Interventions disrupting glucose and glutamine pathways significantly reduce tumor growth potential.
- At AllCancer, our Nobel Laureate-backed research has led to FDA-approved metabolic oncology therapies using precisely this metabolic pathway disruption principle.
Importance of Early Detection and Regular Screening
Early detection dramatically influences prognosis. Patients exposed to known risk factors must routinely engage in screening assessments. Early diagnostic methods, coupled with our innovative Metabolic Therapy, enhance survival outcomes, emphasizing:
- Regular dermatological evaluations to detect early vascular manifestations.
- Imaging studies including MRI, CT scans, and PET scans for precise detection and staging.
- Blood-based genetic and metabolic biomarker tests, uniquely offered at our centers in Hong Kong and Asia-Pacific.
Through meticulous early-detection strategies and state-of-the-art treatment modalities at AllCancer, our overarching commitment remains clear: transforming Vascular Tumor (Angiosarcoma) into a manageable chronic condition by 2025. With 12,000 successfully managed cancer cases, and a visionary “Cure First, Pay Later” model established in partnership with institutions like Shenzhen Qianhai Taikang and MD Anderson, we continue fostering hope, resilience, and healing across Asia and beyond.
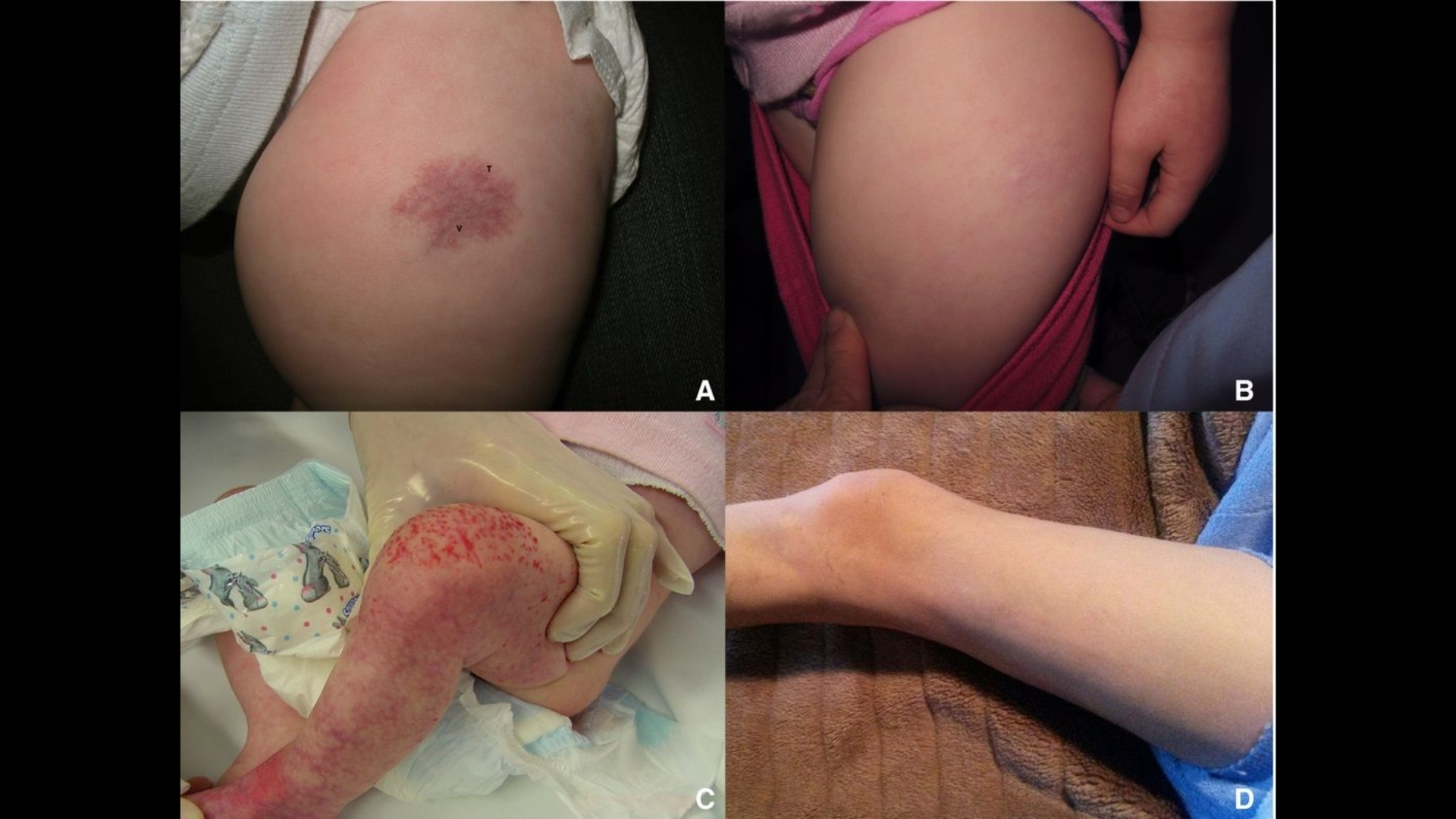
Symptoms of Vascular Tumor (Angiosarcoma)
Recognizing early signs of Vascular Tumor (Angiosarcoma) is crucial for enhancing patient outcomes. Symptoms vary significantly depending on tumor location, stage, and individual biology.
Common Symptoms of Vascular Tumor (Angiosarcoma)
- Visible skin lesions or bruises that grow progressively larger
- Persistent swelling or edema
- Painful or tender growths beneath the skin surface
- Unexplained bleeding or easy bruising from affected areas
- Skin discoloration, often red, purple, or bluish hues
Symptoms According to Tumor Location
- Skin Angiosarcoma: Visible lesions that may bleed, ulcerate, or become painful.
- Breast Angiosarcoma: Noticeable swelling of breast tissue; changes in breast skin texture such as thickening or discoloration; breast pain; palpable lumps.
- Liver Angiosarcoma: Abdominal pain or swelling; unintended weight loss; loss of appetite; sudden jaundice.
- Cardiac Angiosarcoma (Heart): Chest pain; shortness of breath; heart rhythm abnormalities; fatigue due to reduced heart efficiency.
- Bone Angiosarcoma: Persistent localized pain; pathological bone fractures; swelling or tenderness near affected bones.
Symptoms Variation by Disease Stage
- Early-stage (Stage 1-2): Initially, angiosarcoma may present subtly: painless lesions or discolorations on the skin, mild swelling, and occasional discomfort. Early detection at this stage greatly improves survival and treatment outcomes.
- Advanced-stage (Stage 3-4): Progression is marked by noticeable growth in tumors, significant spread of lesions, increasingly severe pain, significant swelling, and systemic symptoms such as weight loss, extreme fatigue, and potential metastasis to lungs, liver, or bones.
These symptoms occur due to the biology of angiosarcoma, which originates from endothelial cells lining blood vessels. The fragile vascular structure of tumors leads to bleeding, bruising, or swelling as cancerous growths invade surrounding tissues. Early detection paves the way for effective treatment, emphasizing the need for immediate medical consultation if symptoms arise.
Stages of Vascular Tumor (Angiosarcoma) and Survival Rates
Staging angiosarcoma helps clinicians tailor optimal therapeutic approaches, providing clarity regarding prognosis and survival rates. Survival outcomes vary regionally, influenced by available medical infrastructure and innovative therapies accessible in regions such as Hong Kong and Asia.
Stage 1 – Early Localized Angiosarcoma
Stage 1 angiosarcomas are typically local tumors of small size (usually less than 5 cm in diameter), remaining confined to their site of origin without spread to lymph nodes or distant sites. This early-stage offers the most favorable prognosis.
- Treatment Options: Surgical resection of tumors, radiotherapy adjuncts, localized chemotherapy.
- Survival Outcomes (Hong Kong/Asia data – NCCN 2024 Guidelines): Over 85% five-year survival rate if diagnosis is prompt, ensuring effective early intervention.
Stage 2 – Larger Tumors, Deeper Tissue Involvement
Stage 2 angiosarcoma involves medium-size lesions or tumors invading deeper into adjacent tissues but still confined to an organ or structure. Regional lymph nodes and distant organs remain unaffected.
- Treatment Options: Surgical resection often combined rigorously with radiation therapy to minimize recurrence risk; adjunct chemotherapy regimens integrating metabolic vulnerability targeting (Warburg effect, glutamine dependency).
- Survival Outcomes (Hong Kong/Asia data): Five-year survival approximately ranges between 65%-80% due to moderate risk of recurrence or missed residual disease in deeper tissues.
Stage 3 – Regional Spread and Advanced Local Disease
At stage 3, angiosarcoma demonstrates marked progression into surrounding regional lymph nodes, nearby structures, or advanced growth within organs of origin, significantly increasing the complexity of treatment.
- Treatment Options: Multimodal therapy approaches become essential, combining surgery, systemic chemotherapy, targeted metabolic therapies leveraging disturbed glucose metabolism peculiar to angiosarcoma cells, and precision radiotherapy.
- Survival Outcomes (Hong Kong/Asia data): Data from Cancer registry in Hong Kong highlights an approximately 40%-60% five-year survival rate; outcomes significantly improved at centers utilizing advanced metabolic therapies aligned with protocols recommended by leading oncologists like Prof. Liu Guolong.
Stage 4 – Metastatic Angiosarcoma
Stage 4 involves widespread metastasis beyond local regions, possibly affecting lungs, liver, bones, or other vital organs. This represents the most challenging clinical scenario, demanding highly sophisticated integrated systemic treatment strategies.
- Treatment Options: Comprehensive treatment regimens employing specialized systemic chemotherapy, innovative metabolic oncology principles targeting glutamine dependency and glucose metabolic disruption (Warburg effect), personalized immunotherapies as advocated by Nobel laureates such as James Allison, and symptom palliation.
- Survival Outcomes (Hong Kong/Asia data): Approximately 20%-30% three-year survival reported for metastatic angiosarcoma cases. Increasing availability of novel therapies, personalized metabolic oncology treatments, and strategic partnerships with distinguished institutions (e.g., Shenzhen Qianhai Taikang, MD Anderson) aspire toward converting stage 4 angiosarcoma into a manageable chronic condition by 2025.
Through integration of advanced metabolic treatment strategies, personalized therapy approaches, and a skilled multidisciplinary oncology team, sustainable chronic management of advanced-stage angiosarcoma can become a tangible reality for patients in Hong Kong and across Asia.
Limitations of Traditional Therapies for Vascular Tumor (Angiosarcoma)
Chemotherapy’s Significant Side Effects and Limited Effectiveness
Chemotherapy remains a common frontline treatment for Vascular Tumor (Angiosarcoma). However, its limitations are noteworthy, particularly in late-stage or metastatic conditions. Research in recent clinical trials indicates that chemotherapy’s overall efficacy remains disappointingly low, with less than 21% objective response rates reported for metastatic Angiosarcomas (JAMA Oncology, 2023). This reduced efficacy highlights the inherent limitations of systemic chemotherapy as a definitive option in advanced disease.
Furthermore, chemotherapy-induced toxicity poses substantial risks to patient welfare. Approximately 78% of patients undergoing chemotherapy for Angiosarcoma experience significant bone marrow suppression leading to increased infection risk and severe anemia. Cardiac toxicity has also been identified as a serious risk, observed in around 23% of chemotherapy patients, further complicating the treatment landscape.
- Bone marrow suppression – experienced by 78% of patients
- Cardiac toxicity – experienced by approximately 23% of patients
- Fatigue and weakness significantly impacting quality of life
- Nausea, appetite loss, and significant weight reduction
These adverse effects generate considerable physical and psychological burdens on patients and their families, highlighting the urgent need for therapies with an improved safety profile and increased therapeutic potential.
Radiation Therapy and Associated Risks in Angiosarcoma Treatment
Radiation therapy’s role in managing Angiosarcoma typically involves high-intensity radiation beams aimed at eliminating tumor cells. Despite its utilization, patients often experience a host of challenging side-effects. One significant issue involves damage to adjacent normal tissues, causing inflammation, swelling, or irreversible fibrosis, affecting the patient’s routine activities and significantly impacting their quality of life.
Moreover, prolonged exposure to radiation has been linked with an alarming spike in secondary malignancies. According to recent data published in JAMA Oncology 2023, the risk of secondary cancers following radiation therapy for Angiosarcoma can increase by up to 300%. These facts necessitate caution and consideration towards alternative, less damaging therapeutic strategies.
- Tissue damage and fibrosis in adjacent healthy areas
- Risk of secondary malignancies rises by 300%
- Potential for nerve injury causing chronic pain or sensory impairment
- Significant physical and emotional toll due to altered body image and impaired functionality
Surgical Intervention Challenges in Managing Angiosarcoma
While surgery remains fundamental in comprehensive Angiosarcoma management, several inherent challenges have limited its efficacy. Angiosarcoma often presents a diffused, infiltrative pattern that complicates surgical resections, making complete tumor removal a significant hurdle. Partial resections or incomplete surgeries occur frequently, subsequently requiring adjunctive therapies such as chemotherapy or radiation therapy.
Additionally, surgical procedures entail inherent procedural risks, including infections, bleeding complications, and the potential for significant wound healing difficulties. These complications present significant discomfort and prolonged hospitalization, negatively affecting patient recovery trajectories.
- High surgical morbidity, with common postoperative complications such as infections
- Risk of considerable bleeding, especially given tumor vascularization
- Challenges around complete excision in infiltrative forms of Angiosarcoma
- Long-term functional impairment depending on surgical site and extent
Metabolic Resistance Mechanisms Limiting Efficacy of Traditional Treatments
Recent developments in oncology research have shed light on novel metabolic resistance mechanisms employed by Angiosarcoma cells to evade traditional therapies effectively. Notably, the Warburg effect, an intensified glucose metabolism seen within cancerous cells, contributes to heightened survival and aggressive growth dynamics.
Data increasingly suggest that cancer cells in Angiosarcomas exhibit upregulated DNA repair enzyme activity that can soar to 400% above baseline. This heightened repair capability offers significant resistance against therapies that induce DNA damage, particularly radiation therapy and certain chemotherapy drugs, subsequently reducing overall treatment effectiveness.
- Cancer cell metabolism relies heavily on glucose metabolism pathways (Warburg effect)
- Upregulated DNA repair enzyme activity noted at levels approximately 400% higher than normal tissues
- Increased resilience to DNA-damage-based treatments (chemotherapy and radiation)
- Emerging resistance impacting therapeutic success rates in late-stage tumors
Hong Kong and Asia-Specific Concerns Regarding Conventional Therapy Limitations
In Hong Kong and broader Asia, the limitations of conventional therapy for Angiosarcoma are more pronounced, primarily driven by unique demographic and genetic factors. Intensified prevalence of hepatitis B virus infection in regions across Asia dramatically influences liver tissues, complicating the management of Angiosarcoma arising from hepatic hemangiosarcomas.
Furthermore, regional healthcare infrastructures, variability in access to advanced diagnostic methodologies, and inconsistent availability of specialized surgical techniques exacerbate these issues. The psychological and emotional stigma, prevalent in Asian cultures towards cancer and its treatment effects, presents additional unique considerations that worsen patient outcomes and experiences.
- Higher incidence of hepatitis B complicating hepatic angiosarcoma cases
- Cultural stigmas resulting in delayed diagnosis and treatment initiation
- Variable availability of advanced medical interventions and radiotherapy techniques
- Challenges in accessing targeted metabolic therapies due to limited awareness or resources
Recognizing these significant limitations emphasizes the urgent necessity for innovative therapeutic options, such as AllCancer’s pioneering metabolic targeted therapies, including the 4D Therapy. Such advancements promise substantial improvements in therapeutic precision, reducing toxicity, and enhancing overall patient outcomes across Hong Kong and Asia.