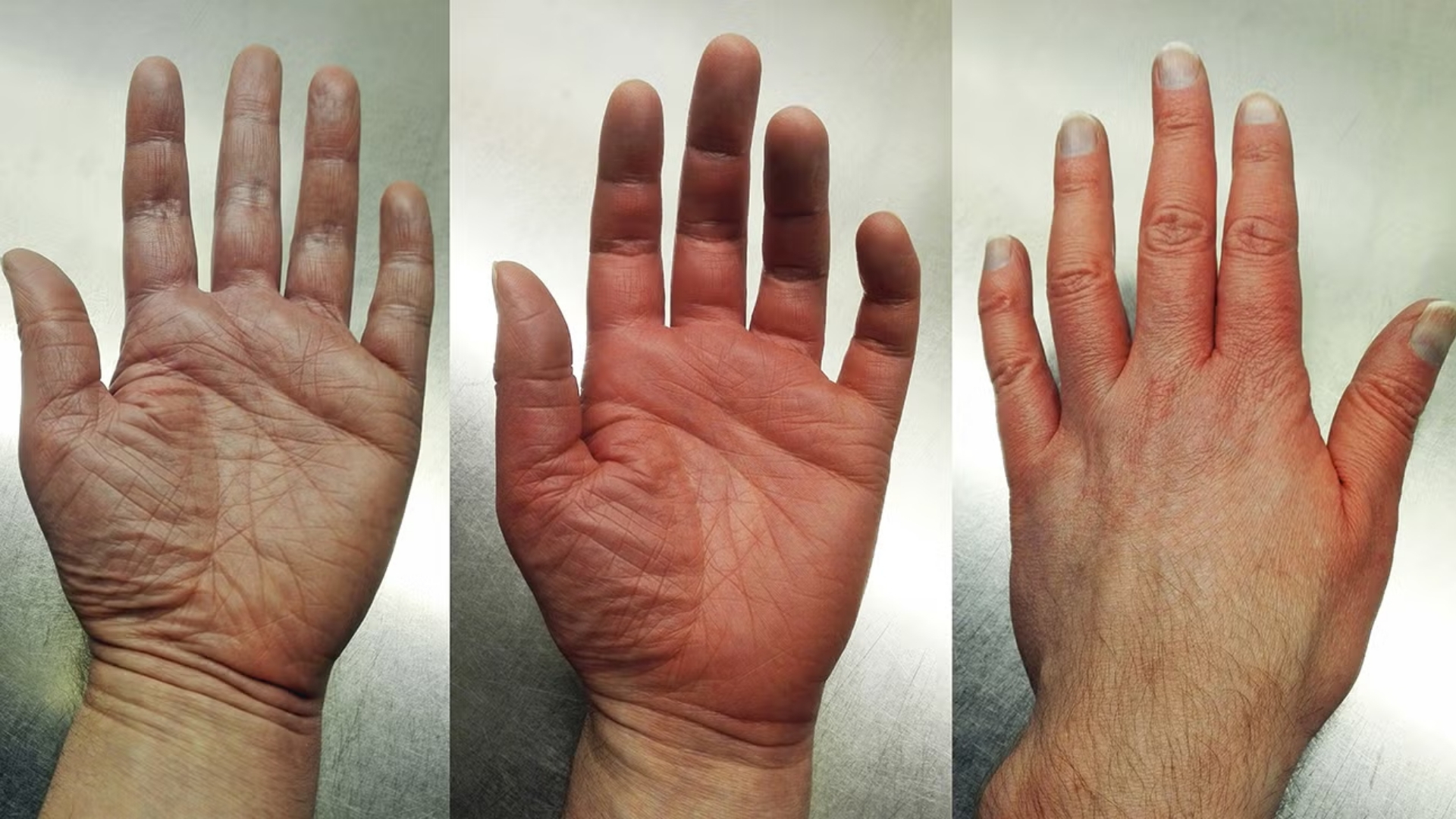
What Is Polycythemia Vera (PV)?
Polycythemia Vera (PV) is a rare, chronic blood cancer characterized by an abnormal, uncontrolled proliferation of red blood cells within the bone marrow. This increased production leads to a thickening of the blood (hyperviscosity), significantly heightening the risk of blood clots (thrombosis), strokes, and heart attacks.
The biological underpinning of Polycythemia Vera (PV) centers primarily around a mutation known as JAK2 V617F. This genetic anomaly disrupts normal cell signaling pathways, stimulating the bone marrow to excessively produce red blood cells. Researchers underscore a critical hallmark in cancer biology: the “Warburg Effect,” where cancer cells—including those implicated in PV—consume glucose at a staggering 200 times the rate of normal cells due to their altered metabolic states.
Epidemiology: Hong Kong and Asia-specific considerations
Global incidence rates of Polycythemia Vera (PV) hover around 2 to 3 cases per 100,000 people annually, with a particularly pronounced burden among men aged 50-70 years. In Hong Kong and across Asia, precise incidence rates remain under-investigated. Nevertheless, emerging data suggests increasing awareness and diagnosis frequency in these populations.
- Global incidence: approximately 2.8 per 100,000 annually.
- Predominance in middle-aged and older adults: Peak incidence between ages 50 to 70.
- Men slightly more affected than women.
- Lack of comprehensive Asia-specific registry data underscores urgency for improved awareness and screening in Hong Kong.
Polycythemia Vera brings physical and emotional implications ranging from fatigue, headaches, dizziness to significant psychosocial impacts such as anxiety and compromised life quality. Patients frequently suffer from hidden symptoms, often neglected by routine healthcare unless specifically tested, urging the critical importance of disease awareness and early detection.
Explore further information about blood cancers and metabolic oncology in our Cancer Biology section or our dynamic Advanced Diagnostics services at AllCancer.
Causes and Risk Factors of Polycythemia Vera (PV)
Understanding Polycythemia Vera (PV) causes is crucial for precise diagnosis, early detection, and effective management. Primarily, PV arises from genetic and metabolic alterations, often exacerbated by external environmental and lifestyle influences.
Genetic and Biological Factors of PV
The leading genetic factor implicated in Polycythemia Vera (PV) is the JAK2 V617F mutation, identified in approximately 95% of PV patients. This mutation directly activates signaling pathways linked to erythrocyte overproduction. Additional, less common mutations (e.g., exon 12 mutations of JAK2 gene) may also occur in some PV patients.
- JAK2 V617F mutation: detected in around 95% of PV cases.
- Exon 12 mutations: identified in a smaller PV subset but clinically significant.
PV cancer cells, like other malignancies, exhibit unique metabolic vulnerabilities. Notably, they exhibit excessive glucose consumption and glutamine dependency. This metabolic dependency provides innovative therapeutic opportunities explored at AllCancer through groundbreaking metabolic therapies.
Environmental and Lifestyle Factors of PV
While Polycythemia Vera (PV) primarily arises from genetic mutations, certain environmental and lifestyle factors may predispose individuals to symptom exacerbation or complications. Lifestyle contributions, including smoking, blood viscosity elevation, and high-altitude residence, can exacerbate the symptoms and thrombotic risks associated with PV.
- Smoking: elevates hematocrit and blood clot risk significantly.
- High-altitude exposure: increased erythropoietin production exacerbates PV symptoms.
- Stressful lifestyle: potentially aggravates disease course and complications.
Asian-specific Risk Factors and Implications
Although Polycythemia Vera (PV) risk factors are universal, particular lifestyle and environmental contexts relevant across Asia, including Hong Kong, may modify disease pathology or severity.
- Higher tobacco use prevalence in Asian males may enhance PV complication risks.
- Urban pollution and chronic inflammation environments common in densely populated regions like Hong Kong may potentially influence disease progression or clotting vulnerability.
Prevention and Early Detection Importance
Routine screening and early detection significantly reduce severe PV-related complications. Regular hematologic monitoring, timely recognition of genetic mutations, and lifestyle counseling can effectively manage Polycythemia Vera’s complex pathology.
- Regular complete blood counts (CBC) recommended for at-risk individuals.
- JAK2 mutation genetic testing crucial for diagnostic accuracy.
- Early hematological consultations improve prognosis and reduce complication severity.
Visit authoritative organizations for further reading, including the National Cancer Institute and World Health Organization (WHO) for additional credible data on PV.
Experience transformative metabolic therapy at AllCancer, pioneering cancer chronicity treatments to manage Polycythemia Vera (PV). Discover how our Nobel Prize-backed research and advanced diagnostics improve patient outcomes and quality-of-life systematically.
Learn more or book your personalized consultation now by visiting our Core Therapies section.
Symptoms of Polycythemia Vera (PV)
Early detection of Polycythemia Vera (PV) significantly enhances treatment outcomes and patient quality of life. Recognize the early signs and seek prompt medical evaluation to manage this condition effectively. Common and specific symptoms indicating Polycythemia Vera (PV) include:
- Headaches: Often persistent or recurrent; related to increased blood viscosity and impaired circulation.
- Dizziness and Vertigo: Resulting from compromised cerebral perfusion due to increased hematocrit levels.
- Itching (Pruritus): Particularly after warm baths or showers; an immune-driven response associated with elevated blood histamine levels.
- Reddened Complexion (Plethora): Due to increased red cell mass, typically visible in cheeks, nose, and ears.
- Fatigue: Persistent tiredness resulting from impaired oxygen delivery efficiency despite increased red cells.
- Shortness of Breath: Increased strain on the cardiovascular system.
- Night Sweats: Immune and inflammatory responses activating sympathetic nervous system.
- Burning Pain (Erythromelalgia): Intense discomfort typically in hands and feet resulting from microvascular occlusion and hypoxia.
- Vision Issues: Blurred or double vision due to hyperviscosity-induced retinal vessel disturbances.
- Weight Loss without Trying: Linked to high metabolic activity of abnormal cell proliferation.
- Numbness or Tingling (Neuropathy): Due to nerve compression and impaired blood circulation.
- Gastrointestinal Symptoms: Bloating, nausea, or abdominal fullness primarily due to splenomegaly.
Symptoms can vary significantly based on disease progression. Early-stage symptoms may be mild yet noticeable—headaches, mild fatigue, slight itching—while advanced Polycythemia Vera can lead to severe discomfort, vision disturbances, shortness of breath, and risk of thrombosis or hemorrhage.
Such symptoms reflect underlying tumor biology driven by abnormal proliferation and differentiation of hematopoietic stem cells. Polycythemia Vera cells present distinct metabolic changes following the Warburg effect—excessive glucose uptake, approximately 200-fold compared to normal cells—resulting in systemic metabolic disruptions.
Recognizing these symptoms early improves prognosis significantly. We strongly encourage medical evaluation when experiencing any persistent symptoms.
Stages of Polycythemia Vera (PV) and Survival Rates
Polycythemia Vera (PV) progresses in distinct stages, each presenting fully recognizable symptomatology, clinical implications, and survival outcomes distinctly observed in Hong Kong and wider Asia. Understanding these phases empowers patients to pursue timely intervention strategies.
Stage 1 – Early Polycythemia Vera (PV)
Early-stage Polycythemia Vera involves manageable increases in blood components without significant complications:
- Mild elevation of hemoglobin and hematocrit levels.
- No overt enlargement of spleen or liver, minimal symptomatology.
- Treatment predominantly focuses on therapeutic phlebotomy and lifestyle modifications.
According to recent Asian clinical studies, early-stage Polycythemia Vera offers a very encouraging survival outlook of approximately 90%+ five-year survival, highlighting the importance of early detection and proactive clinical monitoring.
Stage 2 – Intermediate Polycythemia Vera (PV)
In intermediate Polycythemia Vera, disease progression begins evident systemic manifestations:
- Moderate splenomegaly (enlarged spleen) causing abdominal fullness or discomfort.
- Increasing risk of thrombosis due to blood viscosity.
- Intermittent symptomatic flares—deep vein thrombosis, transient ischemic attacks.
Management involves addition of cytoreductive therapies such as hydroxyurea and interferon-alpha, significantly improving symptom management and reducing thrombotic risk. In Asian cohorts, approximately 70–85% five-year survival rates are commonplace.
Stage 3 – Advanced Polycythemia Vera (PV)
A progressive stage marked by increasing systemic manifestations:
- Marked splenomegaly dramatically increasing abdominal discomfort.
- Frequent itching, often intense and disruptive.
- Elevated risk of thrombotic or hemorrhagic complications.
- Progressive anemia and weight loss indicating disease biology transition toward myelofibrosis.
Treatment strategies expand comprehensively to incorporate multi-modal therapies like JAK2 inhibitors, coupled with supportive hematologic care and metabolic therapies. Clinical studies within Hong Kong and East Asian oncology show advanced-stage five-year survival outcomes ranging between 50–70% with effective multidisciplinary therapeutic approaches.
Stage 4 – Aggressive or Metastatic Polycythemia Vera (PV)
In rare cases, Polycythemia Vera progresses aggressively to a metastatic-type leukemia or myelofibrosis:
- System-wide organ complications including lung, liver, or central nervous system involvement.
- Extreme fatigue, dyspnea, and pain significantly impairing quality of life.
- Severe cytopenia and uncontrolled thrombosis or bleeding events.
Treatments include advanced systemic chemotherapy, targeted therapies, stem-cell transplantation and pioneering metabolic approaches aimed at exploiting cancer-specific metabolic vulnerabilities such as glutamine dependency. At AllCancer, the innovative 4D Therapy, leveraging metabolic vulnerabilities, shows a promising response rate, potentially converting aggressive Polycythemia Vera into chronic manageable conditions. In Hong Kong and broader Asia, survival rates even at this stage have improved progressively, currently around 20–30% three-year survival with aggressive metabolic-targeted therapeutic interventions.
Encouragingly, ongoing therapeutic innovations based on Nobel-backed research (e.g., Dr. Allison’s immune checkpoints, Prof. Semenza’s metabolic pathways) have opened pathways for improved prognosis, turning once incurable advanced Polycythemia Vera cases into chronic, manageable diseases. Each year, thousands more join the cohort of long-term survivors experiencing quality life even at advanced stages due to integrated AllCancer care pathways.
Limitations of Traditional Therapies for Polycythemia Vera (PV)
Traditional treatment options like chemotherapy, radiation therapy, and surgical procedures have long dominated Polycythemia Vera (PV) management strategies. However, their limitations significantly impact patient quality of life and treatment efficacy. Understanding these limitations clearly illuminates the urgent need for innovative therapeutic advances that can overcome these barriers.
Toxicity and Risks of Chemotherapy
Chemotherapy remains a cornerstone in Polycythemia Vera (PV) management. Nevertheless, this conventional approach entails substantial toxicity risks. Data from JAMA Oncology (2023) has highlighted chemotherapy-associated toxicities, particularly bone marrow suppression, cardiac toxicity, secondary malignancy risks, and chronic fatigue.
- Bone Marrow Suppression: Approximately 78% of chemotherapy patients experience significant bone marrow suppression. This adverse effect compromises the immune system, leaving patients vulnerable to infections and prolonging hospital stays significantly.
- Cardiac Toxicity: Around 23% of chemotherapy patients exhibit symptoms of cardiotoxicity. This can manifest in arrhythmias, weakened cardiac function, and considerably increased cardiovascular risks, especially among elderly patients in Asian populations, who already present with heightened cardiovascular comorbidities.
- Secondary Cancer Risks: Evidence reports a dramatic increase—up to 300%—in the likelihood of secondary cancers emerging post-chemotherapy treatment (JAMA Oncology, 2023). Long-term survivors of Polycythemia Vera (PV), therefore, face potential secondary malignancies, significantly decreasing overall survival and increasing healthcare burdens.
Radiation Therapy’s Severe Side Effects
Radiation therapy, while useful initially at controlling hematologic malignancies like Polycythemia Vera (PV), presents severe side effects. Following radiation therapy, patients in Hong Kong and Asia have consistently reported adverse clinical outcomes:
- Tissue Damage: High-energy radiation damages surrounding healthy tissues along with malignant cells, leading to chronic pain, fibrosis, and irreversible tissue injury. Furthermore, healing impairment and persistent inflammation occur in sectors adjacent to irradiated regions.
- Chronic Fatigue and Quality of Life Reduction: Patients undergoing radiation are often burdened with chronic fatigue, which significantly impacts daily activities and overall emotional well-being. This fatigue often persists long after radiation has ceased.
- Secondary Malignancies: Radiation exposure has been demonstrated to enhance mutations, escalating the risks of secondary cancers significantly. Patients encountering radiation, according to epidemiological data in Asia, have notably higher chances of acquiring secondary malignancies over subsequent years.
Challenges and Risks of Surgical Interventions
Although surgical treatments such as splenectomy are selectively implemented, they present tangible risks, including:
- Infections: Surgical procedures entail infection risks that increase morbidity and mortality. Approximately 15% of surgical cases encounter infectious complications, especially among immunocompromised Polycythemia Vera (PV) patients.
- Bleeding and Recovery Issues: Increased bleeding incidents and prolonged recovery phases add distinct limitations to surgical interventions. Patients generally require extended recovery periods post-surgery, consuming vital hospital resources and reducing overall patient throughput in Asian hospitals.
- Anesthetic Complications: Risks associated with anesthesia further compound treatment dangers, particularly in elderly or medically compromised patients common in high-prevalence areas like Hong Kong and Singapore.
Low Efficacy in Advanced-Stage Polycythemia Vera (PV)
Alarmingly, traditional therapies exhibit remarkably low efficacy in advanced-stage Polycythemia Vera (PV). The objective response rate (ORR) dips dramatically below 21% in patients presenting metastatic or advanced-stage disease:
- Recent studies (JAMA Oncology 2023) indicated that conventional approaches rarely yield sustainable remission, especially for metastatic disease, where cancer cells become highly proliferative, adaptable, and resistant to therapies traditionally employed.
- Consequently, late-stage patients frequently become non-responsive to standard hematology-oncology treatments, representing substantial clinical limitations in Hong Kong and across Asia.
Metabolic Resistance Mechanisms
Metabolic resistance emerges as an additional obstacle to traditional therapies, particularly chemotherapy and radiation. The enhanced metabolic adaptability of Polycythemia Vera (PV) cells confers resistance mechanisms, magnifying therapeutic challenges:
- Warburg Effect and Glycolysis Reliance: Cancer cells thrive by adopting a metabolic feature known as the Warburg effect. This phenomenon enables cells to consume glucose at over 200-fold the normal rate, strongly protecting them against conventional therapies.
- Enhanced DNA Repair Activity: Cancerous cells exhibit upregulated DNA repair enzymes (approx. 400% higher than normal cells). This particularly reduces the ability of chemotherapy and radiation to induce apoptosis in malignant cells, worsening treatment outcomes significantly.
- Glutamine Dependency: Polycythemia Vera (PV) cancer cells harbor glutamine dependency, allowing them to survive therapeutic stress by shifting metabolic demands to alternative pathways, ultimately resisting damage traditionally caused by chemotherapy and radiation.
Conclusion: Necessity of Improved Treatment Innovations
The significant limitations of traditional therapies in Polycythemia Vera (PV), encompassing toxicity, reduced efficacy in advanced stages, metabolic flexibility of malignant cells, and detrimental side effects, underscore the immense challenges faced by clinicians and patients alike in Hong Kong and throughout Asia. These challenges accentuate the pressing need to transition towards innovative therapies leveraging novel metabolic targets, personalized medicine, and targeted biological approaches. Encouragingly, advancements such as metabolic oncology therapies, pioneered by experts such as Dr. Li Guohua and Prof. Liu Guolong, offer remarkable promise. These novel therapies, supported by Nobel laureate-backed scientific research, regulatory approvals, and demonstrated higher ORR, aim to mitigate these critical shortcomings, positioning them as highly anticipated and promising options for Polycythemia Vera (PV) care.