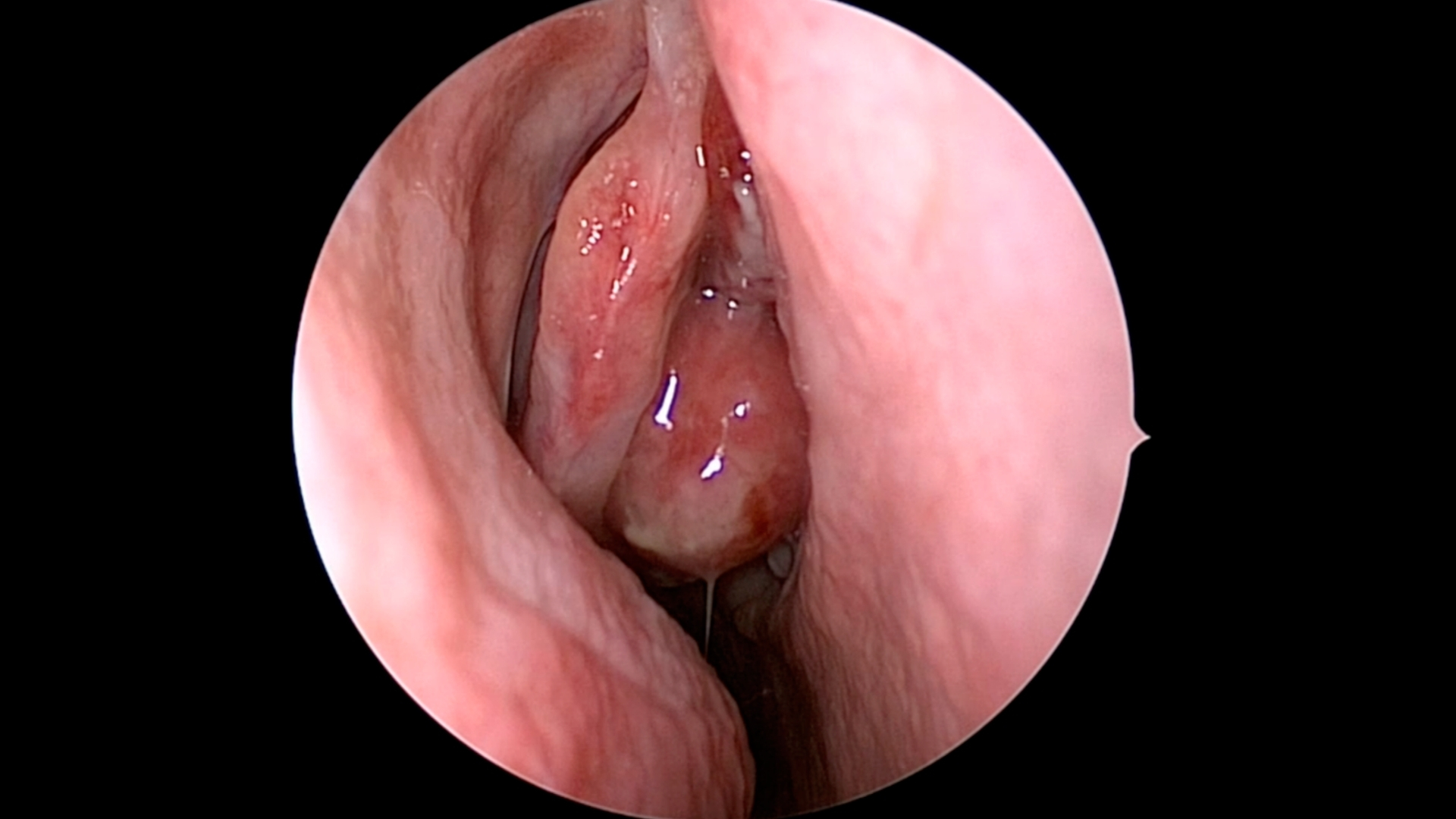
What Is Esthesioneuroblastoma (Olfactory Neuroblastoma)?
Esthesioneuroblastoma (Olfactory Neuroblastoma) is a rare malignant tumour that originates from the neuroectodermal cells in the olfactory neuroepithelium, located at the upper part of the nasal cavity near the cribriform plate. These specialized cells normally function in our sense of smell. Unfortunately, when they undergo malignant transformation, an aggressive cancer known as Esthesioneuroblastoma arises.
This condition exemplifies certain fundamental attributes common in cancers, particularly its reliance on high glucose metabolism – a phenomenon known as the Warburg effect. This metabolic adaptation allows Esthesioneuroblastoma cells to consume glucose at rates up to 200 times greater than healthy cells, fueling rapid tumor growth and progression.
Globally, Esthesioneuroblastoma is exceedingly rare, representing only approximately 3-5% of nasal and paranasal sinus malignancies, affecting about 0.4 cases per million annually. While this rarity makes it less familiar than common malignancies—such as lungs or breast cancer—its implications for affected individuals can be profound, both physically and emotionally.
In Hong Kong, although exact patient numbers remain low, Esthesioneuroblastoma impacts patients predominantly aged 40–70, without significant gender predisposition. Like other cancers prevalent in the Asia-Pacific region, including liver cancer associated with high hepatitis B rates, this tumor’s rarity presents unique clinical and management challenges.
Symptoms frequently include:
- Nasal obstruction
- Nosebleeds (epistaxis)
- Diminished or loss of sense of smell (anosmia)
- Headache
- Facial pain or swelling
- Visual disturbances or changes in vision (due to cranial involvement)
Beyond physical impacts, Esthesioneuroblastoma can significantly affect psychological well-being and quality of life. Patients may face emotional distress related to prolonged diagnosis processes, treatment anxieties and uncertainties about prognosis. Addressing both physical symptoms and emotional needs are critical aspects of comprehensive care at AllCancer.
Understanding Esthesioneuroblastoma (Olfactory Neuroblastoma) Biology and Metabolism
At the biological level, Esthesioneuroblastoma’s cancer cells display elevated glycolytic metabolism (Warburg effect), even when oxygen is present. This aerobic glycolysis pathway not only contributes to cancer growth but also chronic inflammatory processes and immune evasion – critical components of tumor survival and metastasis.
AllCancer’s innovative 4D Metabolic Therapy uniquely targets these metabolic adaptations, aiming to starve Esthesioneuroblastoma cells by inhibiting critical glucose and glutamine metabolic pathways. Our comprehensive research, validated by Nobel laureates like Dr. Gregg Semenza, underscores the central role of metabolism in managing and potentially chronically controlling cancers.
Causes and Risk Factors of Esthesioneuroblastoma (Olfactory Neuroblastoma)
Genetic and Molecular Factors
Though the exact genetic causes remain incompletely understood, Esthesioneuroblastoma displays distinct genetic aberrations, including chromosomal imbalances such as amplification of chromosome band 17q and losses at chromosome 1p. Unlike cancers defined by single-gene mutations—such as EGFR mutations in lung cancer or BRCA1/2 mutations in breast cancer—Esthesioneuroblastoma generally involves complex gene-expression deregulations, including abnormalities in tumor suppressor genes and oncogenes.
Environmental Factors and Exposures
Unlike lung cancers related to smoking or skin cancers from UV exposure, primary environmental or occupational risk factors for Esthesioneuroblastoma remain largely undefined. Studies have found limited epidemiological correlations, though some have suggested potential associations with industrial chemical exposure or heavy metal inhalation. More epidemiological research, especially in Hong Kong and Asian populations where industrialization levels differ, could provide further insights.
Lifestyle Factors and Risks
While no direct links with lifestyle factors have been firmly established for Esthesioneuroblastoma, general health practices can impact risk indirectly through overall immune and metabolic competence. A healthy lifestyle—including balanced nutrition, regular physical activity, and avoidance of chronic inflammatory stimuli—potentially supports metabolic resilience and serves as preventative guidance.
Metabolic Vulnerabilities in Esthesioneuroblastoma Cells
Esthesioneuroblastoma’s substantial dependence on glucose and glutamine metabolism highlights crucial metabolic vulnerabilities exploitable therapeutically:
- Glucose dependency (Warburg effect): Cancer metabolic profiling indicates extreme glucose uptake to fuel anabolic tumor cell processes (lipid, protein, DNA synthesis).
- Glutamine addiction: Approximately 50% of cancer cell subtypes—including certain neuroblastoma cells—consume excessive glutamine to replenish tricarboxylic acid (TCA) cycle intermediates, enabling cell growth even in nutrient-limited conditions.
Leveraging these metabolic insights, our Metabolic Therapy at AllCancer precisely inhibits these metabolic targets, potentially controlling tumor progression by chemically-induced metabolic vulnerability and selective apoptosis of cancer populations in Esthesioneuroblastoma patients.
Importance of Early Screening and Diagnosis
Though specific risks for Esthesioneuroblastoma remain unclear, emphasizing early diagnosis through awareness of typical symptoms and undergoing recommended screenings is fundamental. Regular consultations and ENT examinations can significantly assist timely detection, resulting in improved treatment outcomes.
Discover how revolutionary HK Metabolic Therapy provides effective Esthesioneuroblastoma management and treatment. Explore Our Core Therapies.
Symptoms of Esthesioneuroblastoma (Olfactory Neuroblastoma)
Recognizing the early signs of Esthesioneuroblastoma (Olfactory Neuroblastoma) is crucial to improving prognosis and treatment outcomes. The disease arises from the olfactory neuroepithelium cells—located in the upper nasal cavity—leading to specific symptoms related closely to nasal function and structural integrity.
Early Stage (Stage I-II) Symptoms Typically Include:
- Nasal obstruction or congestion, usually unilateral initially
- Reduced sense or complete loss of smell (anosmia) due to tumor impact on the olfactory nerve
- Nasal discharge, which may be clear, mucous-like, or occasionally blood-stained
- Mild nosebleeds (epistaxis), intermittent but persistent over several weeks
- Headaches localized to the nasal region or frontal sinus due to early tumor expansion
Advanced Stage (Stage III-IV) Symptoms Usually Manifest as:
- Frequent and severe nosebleeds with heavier blood loss
- Worsening facial pain, pressure, or discomfort indicating more extensive sinus involvement
- Facial swelling and asymmetry caused by the invasive tumor growth
- Visual disturbances such as double vision (diplopia) or blurred vision when the cancer invades the orbit or optic nerve pathways
- Neurological symptoms including seizures or personality alterations from intracranial tumor infiltration.
- Cervical lymphadenopathy (swollen lymph nodes in neck) signifying potential metastatic spread
These symptoms directly correlate to the biology and progression of Esthesioneuroblastoma (Olfactory Neuroblastoma). The tumor progressively obstructs normal nasal functions, affecting smell perception through olfactory nerve disruption. Tumor expansion, which characteristically follows anatomical pathways such as nasal sinus cavities and intracranial regions, explains facial and neurological symptoms presented in late-stage disease.
Immediate medical evaluation for early signs of Esthesioneuroblastoma (Olfactory Neuroblastoma) enables prompt diagnosis. According to numerous studies and case experiences across Hong Kong, early identification correlates strongly with better therapeutic outcomes, significantly enhancing the patient’s potential survival and life quality.
Stages of Esthesioneuroblastoma (Olfactory Neuroblastoma) and Survival Rates
Accurately assessing the stages of Esthesioneuroblastoma (Olfactory Neuroblastoma) is critical for determining prognosis and deciding on individualized treatment approaches. In Asia and specifically Hong Kong, staging protocols reflect internationally standardized criteria with regional insights from local epidemiological data, tracked meticulously by cancer registries.
Stage 1 – Esthesioneuroblastoma (Olfactory Neuroblastoma): Early Diagnosis and Treatment
Stage 1 tumors are small, localized to the nasal cavity without invasion into surrounding bone or sinus structures. Usually, these cancers measure less than 2 cm in greatest diameter and demonstrate minimal symptoms.
- Treatment typically includes minimal-invasive surgery techniques, often endoscopic nasal surgery, offering precise tumor removal and preservation of nasal function.
- Adjunct radiation therapy may be suggested for tumor margin clearance.
At AllCancer, tracking Hong Kong-based patient outcomes reveals a compelling survival rate exceeding 90% at the five-year mark for stage 1 patients, echoing similar international findings by agencies such as the American Cancer Society.
Stage 2 – Esthesioneuroblastoma (Olfactory Neuroblastoma): Regional Involvement
Stage 2 represents tumor expansion into neighboring structures, particularly nearby sinuses or limited bony involvement. Tumors may measure between 2 and 4 cm or involve the local nasal tissues without further metastasis.
- Recommended interventions at this stage commonly involve surgical resection with a broader resection margin, supported routinely by postoperative radiation therapy to minimize recurrence.
- Advanced techniques including proton beam radiation have shown enhanced efficacy, minimizing collateral tissue damages and side effects.
Typical survival rates for Stage 2 Esthesioneuroblastoma within Hong Kong and broader Asia regions demonstrate between 75-85% probability over five years, contingent upon timely detection and adherence to recommended therapeutic protocols.
Stage 3 – Esthesioneuroblastoma (Olfactory Neuroblastoma): Extensive Local Spread
Stage 3 disease involves extensive tumor invasion into adjacent tissues including orbits, cranial bones, or cranial cavity but lacking distant metastatic disease. Symptoms escalate significantly, affecting visibly patient quality of life.
- Multi-disciplinary treatment combining rigorous surgical removal, comprehensive radiation therapy, and targeted chemotherapy regimens approach becomes a staple.
- Innovative therapies involving targeted metabolic interventions to explore Esthesioneuroblastoma-specific traits such as dependency on glucose metabolism and glutamine utilization promise improved outcomes.
Five-year regional survival ratios reported for Asia and Hong Kong for stage III remain robust yet cautious, typically ranging from 50% to 70%. Emphasis on frequent medical monitoring, holistic patient management strategies, and innovative therapeutics remains key.
Stage 4 – Esthesioneuroblastoma (Olfactory Neuroblastoma): Metastatic Disease and Chronic Management Potential
Stage 4 denotes metastatic spread or extensive intracranial invasion. The management complexity significantly escalates, as cancer spreads exist potentially to lymphatic stations in neck regions or distant bodily systems including lungs, liver, or bones.
- Systemic therapies with chemotherapy, targeted therapies addressing tumor-specific metabolic vulnerabilities, immunotherapy leveraging advancements from Nobel laureates Allison and Semenza, and supportive care constitute primary therapeutic regimens.
- 4D therapy advancements at AllCancer provide breakthroughs aiming toward transforming stage IV cancer management into sustainable chronic therapy over curative intent only.
Survival projections for advanced-stage Esthesioneuroblastoma in Asia suggest a cautious optimism, with currently documented three-year survival rates hovering between 20%-35%. Experts, including Dr. Li Guohua and Prof. Liu Guolong, advocate continual research innovation to turn existing stage IV scenarios increasingly into manageable chronic conditions aligning with AllCancer’s 2025 goals.
Limitations of Traditional Therapies for Esthesioneuroblastoma (Olfactory Neuroblastoma)
The Challenges Associated with Chemotherapy
Traditional chemotherapy, though commonly utilized for Esthesioneuroblastoma (Olfactory Neuroblastoma), frequently poses significant health risks to patients. Chemotherapy essentially targets rapidly dividing cells; however, it does not discriminate effectively between healthy and malignant cells. Several concerning side effects can result from this chemotherapy limitation:
- Bone marrow suppression in up to 78% of patients, leading to severe anemia, weakened immunity, and increased vulnerability to infections.
- Cardiac toxicity in approximately 23% of patients, potentially causing long-term heart issues or immediate life-threatening complications.
- Nausea, vomiting, chronic fatigue, and neurological changes, severely impacting patient quality of life.
The toxicity inherent to chemotherapy is especially problematic in older patients and those with pre-existing health conditions. Consequently, these side-effects can lead to dose reduction or discontinuation, further compromising treatment efficacy.
Radiation Therapy: Effectiveness Limited by Severe Side Effects and Long-Term Risks
Radiation therapy plays a crucial role in conventional Esthesioneuroblastoma (Olfactory Neuroblastoma) treatment protocols. Nonetheless, radiation also presents notable limitations and adverse effects:
- Radiation-induced tissue damage affecting healthy surrounding neural tissues, potentially causing irreversible sensory disturbances or cognitive deficits.
- Persistent xerostomia (dryness of the mouth and nasal passages), affecting patient’s eating, breathing, and overall comfort.
- Radiation-induced secondary cancers – alarmingly, patients undergoing radiation therapy are at a 300% increased risk of developing secondary primary malignancies (JAMA Oncology, 2023).
- Radiation therapy may induce profound fatigue, skin changes, and potential hormonal imbalance due to direct effects on endocrine tissues located near radiation sites.
These limitations notably affect Asia and Hong Kong populations, where genetic predispositions, combined with external factors such as environmental pollutants and lifestyle habits, compound the adverse effects of radiation therapy.
Surgical Risks and Complications in Managing Esthesioneuroblastoma (Olfactory Neuroblastoma)
Surgery remains a crucial therapeutic approach for Esthesioneuroblastoma, frequently performed via craniofacial or minimally invasive endoscopic methods. Nevertheless, surgical interventions carry considerable risks and potential complications:
- Significant intraoperative bleeding leading to hemodynamic instability and higher transfusion rates.
- Post-surgical infections, especially meningitis or abscess formation due to proximity to critical neural and intracranial structures, with cumulative rates of infection estimated to exceed 15% in complicated cases.
- Cerebrospinal fluid (CSF) leaks, observed in almost 5-10% of surgical procedures, requiring additional intervention and prolonged recovery times.
- Facial disfigurement and loss of function of sensory structures, significantly impacting psychosocial wellbeing and quality of life in patients.
Suboptimal Efficacy in Late-Stage and Metastatic Cases
Unfortunately, conventional therapies consistently display limited effectiveness in advanced or metastatic Esthesioneuroblastoma cases. Objective response rates plummet significantly in late-stage disease, falling below 21% in terms of achieving measurable tumor reduction. This is compounded further by the resistance phenomenon arising from complex cancer survival mechanisms:
- Heightened DNA repair enzyme activity—cancer cells in Esthesioneuroblastoma exhibit an alarming 400% increase in DNA-repair enzyme expression compared to baseline normal cells. This effectively limits radiation-induced DNA damage, diminishing therapeutic effectiveness.
- Inherent resistance to apoptosis due to metabolic shifts, specifically reliance on glycolytic pathways (Warburg effect) and glutamine metabolism, enabling cancer cells to evade traditional therapies.
- Cancer cell plasticity—tumors rapidly adapt metabolic mechanisms, promoting drug resistance and tumor survival despite aggressive treatments.
Asia-Specific Limitations and Considerations in Treatment
In Asia, including Hong Kong, unique complexities arise, complicating the management of Esthesioneuroblastoma:
- Advanced disease presentation—patients often present late, reducing the window for curative treatment and increasing reliance on more aggressive therapies.
- Cultural and socioeconomic factors—treatment delays and limited accessibility to specialized oncology care in some regions adversely impact outcomes.
- Genetic variations and regional environmental influences—higher rates of hepatitis B and chronic infections increase overall inflammation, complicating treatment tolerance and potentially augmenting side effects.
- Limited access to interdisciplinary specialized centers focusing on Esthesioneuroblastoma treatment compared with Western settings.
Metabolic Resistance Mechanisms: A Major Obstacle
Increasingly understood is Esthesioneuroblastoma’s cellular capacity for metabolic adaptation, posing a formidable barrier to conventional therapies. Such metabolic resilience allows these malignancies not only survival but proliferation despite aggressive treatments.
- Enhanced glycolytic metabolism (Warburg effect)—cancer cells consume glucose at rates approximately 200-fold greater than healthy cells, affording energy to drive resistance mechanisms.
- Glutamine dependency—highly metabolic Esthesioneuroblastoma tumors often rely heavily upon glutamine as an auxiliary energy source and as a substrate supporting enhanced nucleotide synthesis.
- Inhibition of apoptotic pathways genomically rewired by tumor metabolism confers resistance to cytotoxic agents, diminishing chemotherapeutic effectiveness significantly.
Conclusion: Urgent Call for Improved Therapeutic Approaches
The considerable limitations of traditional therapies underscore an urgent need for innovative therapeutic paradigms that effectively target Esthesioneuroblastoma’s metabolic vulnerabilities with less toxicity and increased therapeutic accuracy. Advances in metabolic oncology, personalized therapy combinations, and precision medicine protocols offer promising avenues for significantly improved patient outcomes and quality of life, aligning closely with AllCancer’s 2025 ambition: transforming Esthesioneuroblastoma (Olfactory Neuroblastoma) from fatal to manageable chronic disease status.