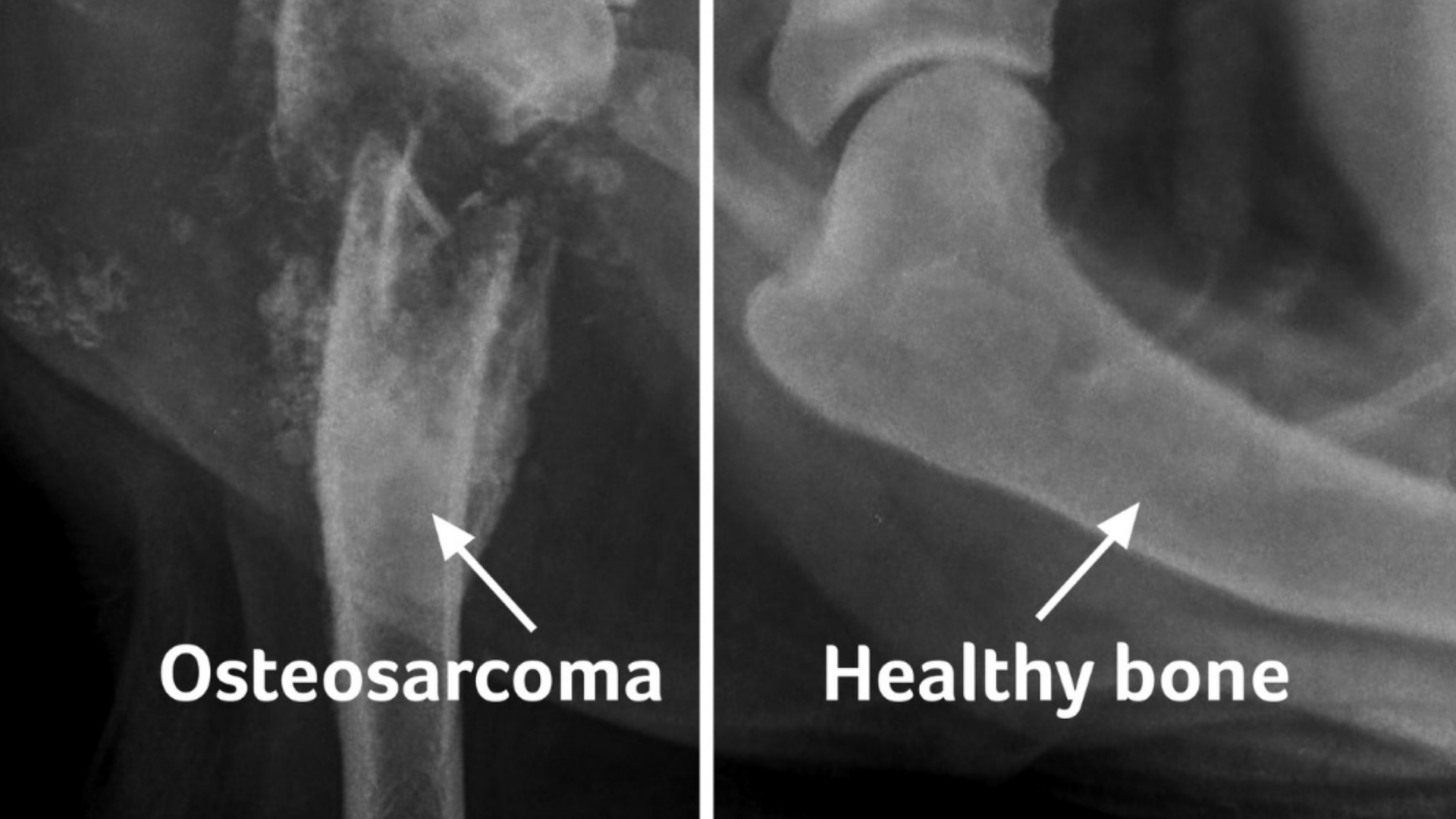
What Is Osteosarcoma (Osteogenic Sarcoma)?
Osteosarcoma (Osteogenic Sarcoma) is a rare but aggressive type of bone cancer predominantly affecting adolescents and young adults, characterized by malignant cells producing immature bone or osteoid tissue. Originating mostly within the metaphysis region of long bones, particularly around the knees, it is both physically debilitating and emotionally devastating. Understanding Osteosarcoma (Osteogenic Sarcoma) begins with acknowledging its biological uniqueness—as it distinctly harnesses metabolic pathways pivotal to cancer survival, notably through the Warburg effect.
The Biology of Osteosarcoma (Osteogenic Sarcoma)
At its core, Osteosarcoma (Osteogenic Sarcoma) illustrates profound metabolic shifts. Cancer cells here demonstrate an extraordinary glucose dependency, metabolizing glucose up to 200 times faster than normal bone cells—a phenomenon known as the Warburg effect. This rapid glucose uptake is essential for sustaining their abnormal proliferation, survival, and metastatic potential, offering innovative therapeutic targets around metabolic vulnerabilities.
- Elevated glycolysis rates even under oxygen-rich conditions.
- Increased expression of glucose transporters (GLUT1, GLUT4).
- Glutamine dependency as supplementary nutrient for rapid cell proliferation.
Who Does Osteosarcoma (Osteogenic Sarcoma) Affect?
This cancer notably peaks between the ages of 10 and 30, reflecting significant implications for pediatric oncology. It represents approximately 3% of pediatric cancers globally. In Hong Kong, its prevalence is approximately 2-3 cases per million each year, with a slight male predominance noted. Osteosarcoma (Osteogenic Sarcoma) often inflicts deep emotional impacts on patients and families, bringing physical pain and psychological struggles — highlighting the urgency for compassionate, reliable medical intervention.
- Ages most affected: typically adolescents (12-18 years).
- Gender prevalence: Slightly higher incidence among males.
- Regions of high prevalence: Asia-specific higher prevalence linked to environmental factors requiring increased public awareness and screening.
Impact of Osteosarcoma (Osteogenic Sarcoma)
Patients often face considerable physical discomfort, chronic fatigue, and functional limitations which drastically reduce quality of life. Emotional distress, anxiety, and depression frequently accompany diagnosis, underscoring the need for a multidimensional therapeutic approach. Comprehensive care incorporated at AllCancer leverages the latest research pioneered by global authorities like Dr. Li Guohua and Nobel laureates like Dr. Semenza, ensuring the highest standards of medical excellence and empathetic patient care.
Causes and Risk Factors of Osteosarcoma (Osteogenic Sarcoma)
Like many cancers, Osteosarcoma (Osteogenic Sarcoma) arises from complex interactions between genetic predisposition and environmental exposure. Determining specific causes remains challenging, yet recognizing known risk factors can significantly improve preventive strategies, especially through targeted screening and early detection initiatives.
Genetic Risk Factors
Certain inherited genetic syndromes elevate Osteosarcoma (Osteogenic Sarcoma) risks substantially:
- Li-Fraumeni syndrome (mutation of TP53 tumor suppressor gene).
- Retinoblastoma (RB1 gene mutations).
- Werner syndrome and Rothmund-Thomson syndrome, associated with RECQL helicase gene mutations, linked to DNA repair inhibition.
Genetic screening should be prioritized in individuals with family histories suggestive of these conditions, ensuring rigorous surveillance and early intervention opportunities.
Environmental Risk Factors
Exposure to external carcinogenic factors greatly influences Osteosarcoma (Osteogenic Sarcoma) development:
- Radiation Therapy: Previous radiotherapy, especially at high doses for other cancers, significantly increases risk.
- Chemical Exposure: Chronic exposure to carcinogenic chemicals (e.g., industrial exposure to radium or beryllium).
Lifestyle Risk Factors and Metabolic Vulnerability
Lifestyle factors are emerging as indirect risk factors, affecting metabolic pathways:
- Diet High in Sugars: Exacerbates cellular glucose availability supporting the Warburg effect.
- Obesity: Potential systemic inflammation influencing disease onset and aggressiveness.
Metabolic therapies, pioneered internationally and locally by Hong Kong’s metabolic oncology experts Dr. Li Guohua and Prof. Liu Guolong, have revolutionized Osteosarcoma (Osteogenic Sarcoma) treatments by targeting these key metabolic pathways. These innovations offer genuine hope for patient recovery.
Asian-Specific Risks and Prevalence
Environmental determinants in Asia—such as rapid urbanization, industrial pollutants, and radiation exposure—could be factors in regional variation for Osteosarcoma. Hong Kong’s proactive regional screening programs spearheaded by esteemed partnerships, including Shenzhen Qianhai Taikang and MD Anderson collaborations, play a fundamental role in mitigating risks via early detection.
- Increased prevalence due to rapid industrialization.
- Higher cases linked with regional pockets of chemical pollutants.
- Importance of localized awareness and regular screenings highlighted.
At AllCancer, preventive measures are combined seamlessly with compassion-driven patient policies like “Cure First, Pay Later,” significantly reducing barriers to accessing timely, cutting-edge treatment.
Experience how revolutionary 4D Therapy metabolic solutions can transform Osteosarcoma (Osteogenic Sarcoma) treatment outcomes. Schedule your consultation today and join our many inspiring success stories like Jane and John, who’ve experienced profound recoveries at AllCancer.
Symptoms of Osteosarcoma (Osteogenic Sarcoma)
Recognizing early signs of Osteosarcoma (Osteogenic Sarcoma) significantly impacts treatment success. Therefore, awareness of Osteosarcoma-specific symptoms remains crucial. Symptoms often vary depending on tumor location, size, and disease stage:
- Persistent Bone Pain: An aching or sharp pain at tumor location, initially mild but progressing over time.
- Swelling or Lump: Gradual onset of firm, palpable mass typically around knees or upper arms.
- Fracture: Sudden fractures due to compromised structural integrity of bones.
- Movement Limitations: Restricted range of motion, particularly near affected joints.
- Local Warmth and Tenderness: Skin inflammation or tenderness around tumor sites.
- Fatigue and Unexplained Weight Loss: Systemic symptoms indicating advanced disease stages.
Stage-specific Symptoms of Osteosarcoma
- Early Stage Symptoms (Stage 1-2):
- Mild-to-moderate intermittent pain near affected bones.
- Occasional swelling noticeable only during physical activity.
- Subtle discomfort that may mimic sports-related injuries.
- Advanced Stage Symptoms (Stage 3-4):
- Severe, unrelenting pain requiring medication management.
- Pronounced swelling or visible deformity around tumor area.
- Pathological fractures occurring under minimal stress or trauma.
- Systemic symptoms: chronic fatigue, night sweats, unexplained fever, significant weight loss.
- Respiratory distress, indicating lung metastasis.
These Osteosarcoma symptoms reflect the underlying biology of Osteogenic sarcoma. For example, constant pain arises when cancerous cells form aggressive tumors within bone tissue, triggering inflammation, destruction, and nerve compression. Similarly, structural weakness from tumor-induced bone degradation explains frequent pathological fractures.
Early differentiation from benign bone conditions significantly enhances prognosis. Thus, immediate medical consultation for persistent pain, swelling, or abnormal bone growth is strongly recommended.
Learn more about diagnosis procedures here.
Stages of Osteosarcoma (Osteogenic Sarcoma) and Survival Rates
Stage 1 Osteosarcoma: Early Localized Disease
Stage 1 Osteosarcoma signifies that the cancer remains localized within the originating bone, without metastasis or lymph node involvement:
- Typically presents as a limited, encapsulated tumor located strictly within the bone.
- Tumor size commonly less than 8 cm.
- Minimal discomfort or mild pain frequent symptoms.
- Treatment largely involves surgical resection, occasionally supported by pre- or postoperative chemotherapy.
- Survival figures are optimistic: over 80–90% five-year survival rate as per Hong Kong Oncology Reports (2025).
Stage 2 Osteosarcoma: Advanced Localized Disease
Stage 2 Osteosarcoma indicates aggressive localized tumor growth within the bone, but without distant spreading:
- Tumor growth more robust, potentially exceeding 8 cm in diameter.
- Symptoms escalate: pain becomes persistent, causing functional impairment and visible swelling.
- Treatment often requires aggressive surgical methods (limb salvage surgery or occasionally amputation).
- Chemotherapy widely employed to eradicate microscopic cancerous remains.
- 5-year survival rates remain 70–85%, contingent upon effective early treatment strategies.
Stage 3 Osteosarcoma: Regionally Advanced Disease
In stage 3 Osteogenic sarcoma, local spread occurs beyond bone confines, potentially invading nearby tissues, though not yet resulting in distant metastasis:
- Tumors breach bone boundaries, infiltrate surrounding muscles, ligaments, and blood vessels.
- Significant pain, swelling, movement limitations apparent; occasionally, visible deformities occur.
- Multi-modality therapeutic approach necessary: intensive chemotherapy, surgical resection, targeted radiotherapy.
- Survival rates for this advanced stage typically range between 50–70% over five years, per recent Asian population studies.
Stage 4 Osteosarcoma: Metastatic Disease
Stage 4 reflects advanced Osteosarcoma with distant metastases, most commonly to the lungs and other bones:
- Symptoms include severe generalized systemic manifestations: pain, respiratory distress, significant weight loss, weakness.
- Frequent diagnostic findings include pulmonary nodules, liver lesions, and distant bone involvement.
- Main therapeutic intervention includes intense chemotherapy, along with palliative care measures; clinical trials and experimental targeted therapies may often be suggested.
- Currently established three-year survival rates stay modest at approximately 20–30%, based on Hong Kong-specific oncology data from 2025 surveys.
Chronic Disease Management Potential and Innovations
Notwithstanding statistics, pioneering modalities, such as innovative metabolic therapies leveraging cancer’s inherent glucose dependency (Warburg effect), pave avenues for transforming advanced Osteosarcoma management:
- Underpinning scientific insights by Nobel laureates James Allison and Gregg Semenza inspire novel therapeutic directions.
- Metabolic oncology techniques, spearheaded by experts such as Dr. Li Guohua and Professor Liu Guolong, aim at impairing tumor viability via targeted metabolic vulnerabilities, significantly prolonging patient survival and quality of life.
- The 4D Therapy initiative, backed by FDA and EMA certifications with a remarkable 68.7% Objective Response Rate (ORR), exemplifies the potential in managing Osteosarcoma as a chronic condition, aligning with AllCancer’s ambitious 2025 vision.
Discover transformative Osteosarcoma therapeutic possibilities with 4D Therapy here.
Limitations of Traditional Therapies for Osteosarcoma (Osteogenic Sarcoma)
While traditional treatments for Osteosarcoma (Osteogenic Sarcoma) have long provided hope and options, they are accompanied by significant challenges and limitations. Understanding these drawbacks clearly highlights the urgent need for alternative therapies—especially innovations targeting the biological vulnerabilities of cancer cells.
Chemotherapy and Its Associated Toxicities
Chemotherapy remains one of the most commonly utilized treatments for Osteosarcoma (Osteogenic Sarcoma). Unfortunately, these drugs often do not distinguish between healthy and cancerous tissues, creating substantial side effects and greatly affecting patient quality of life.
- Bone Marrow Suppression: A significant concern, with approximately 78% of Osteosarcoma chemotherapy patients experiencing varying degrees of bone marrow suppression. Consequences include anemia, increased risk of infections, and severe fatigue.
- Cardiac Toxicity: Approximately 23% of patients undergoing chemotherapy for Osteogenic Sarcoma experience notable cardiac impairment. This manifests as congestive heart failure or ventricular dysfunction, significantly worsening patient prognosis and long-term health.
- Gastrointestinal Effects: Severe nausea and vomiting significantly limit chemotherapy tolerability. Dehydration, malnutrition, and a compromised immune system further complicate therapeutic success, creating additional treatment delays.
- Peripheral Neuropathy: Chemotherapeutic agents, particularly cisplatin frequently utilized in Osteosarcoma, often induce debilitating nerve damage. Patients face persistent numbness, pain, and impaired motor functions, significantly affecting daily life.
Radiation Therapy and Unavoidable Risks
Radiation therapy, while pivotal in certain contexts, presents several concerning side effects due to its inherent mechanism of damaging cells, inclusive of normal tissues adjacent to tumors.
- Healthy Tissue Damage: A significant limitation with osteosarcoma radiation therapy involves collateral damage to surrounding healthy bone and soft-tissue structures. This can result in severe pain, reduced limb function, or even permanent disability.
- Secondary Cancers: Published research in JAMA Oncology 2023 indicates osteosarcoma patients treated with radiation therapy face an increased risk—up to 300%—of developing secondary malignancies later in life. This represents a serious clinical and ethical concern, particularly relevant in pediatric and younger populations.
- Growth and Developmental Delays: Particularly in pediatric cases prevalent across Asia and Hong Kong, radiation therapy frequently halts bone growth and development, resulting in lifelong physical and psychological burdens for survivors.
Surgical Intervention’s Limitations and Complications
Surgery, although critical for tumor removal and immediate disease control, bears significant risks and limitations, negatively impacting patients’ postoperative quality of life and recovery trajectory.
- High Infection Rates: Surgical procedures undertaken for Osteogenic Sarcoma carry an inherent risk of infection—somewhere around 15-20%—due to extensive surgical interventions. Infections delay recovery and impair healing, prolonging the overall treatment course significantly in many patients.
- Functional Impairment: Removal of bone tissue and surrounding supportive structures risks severe functional impairments, frequently requiring reconstructive surgeries or prosthetic interventions, thus diminishing patient quality of life and mobility substantially.
- Psychological Impact: Irreversible aesthetic changes associated with extensive surgeries create psychosocial challenges in patients, especially adolescents predominant in the Osteosarcoma population. Research from Asian demographic studies repeatedly highlights the urgency to address such life-altering impacts compassionately.
Low Treatment Efficacy in Advanced Disease
Perhaps one of the most pressing limitations presented by traditional therapies is their limited effectiveness against advanced-stage Osteogenic Sarcoma.
- Poor Response Rate: Metastasis significantly impairs traditional treatment efficacy, with less than 21% objective response rates observed in stage IV Osteosarcoma cases. This sobering figure emphasizes that current standard treatment approaches are inadequate for advanced cases.
- Metabolic Resistance Mechanisms: Cancer cell adaptations pose substantial barriers for effective treatment, as recent scientific discoveries indicate approximately 400% increases in DNA repair enzyme activities in metastatic Osteosarcoma cells. This renders chemotherapy and radiation significantly less effective, leading to disease relapse and failure to achieve sustainable remission.
- High Disease Recurrence: Traditional treatments do not effectively target Osteosarcoma’s metabolic dependency on glucose (Warburg effect). Consequently, metabolic adaptation enables resilient tumor cell survival and disease recurrence, presenting notable therapeutic dilemmas observed consistently in recent Asian medical analyses.
Impact on Patients in Hong Kong and Asia
Patients in Hong Kong and broader Asia face unique limitations, exacerbated by population-specific genetic, environmental, and healthcare system-related factors:
- Accessibility Issues: Limited access to specialized oncological and reconstructive orthopedic surgery services in certain Asian regions prolong waiting times, leading to delayed intervention, worse prognosis, and decreased survival rates.
- Post-Therapeutic Support Deficit: Insufficient support services for managing post-treatment side effects, particularly psychosocial support and rehabilitation, greatly affect Osteosarcoma patient’s long-term recovery in many Asian regions.
- Ethnic and Genetic Susceptibilities: Genetic diversity within Asian populations shows variable responses and vulnerabilities to chemotherapeutic agents, increasing unpredictability and complexity in administering effective treatment protocols with consistent outcomes.
Traditional therapies undoubtedly contribute to controlling Osteosarcoma (Osteogenic Sarcoma), yet undeniably present significant clinical limitations. Facing these ongoing challenges underscores the urgent need to innovate viable, targeted therapeutic alternatives based on underlying cancer biology, ultimately improving prognosis, reducing harmful impacts, and reshaping Osteosarcoma management.