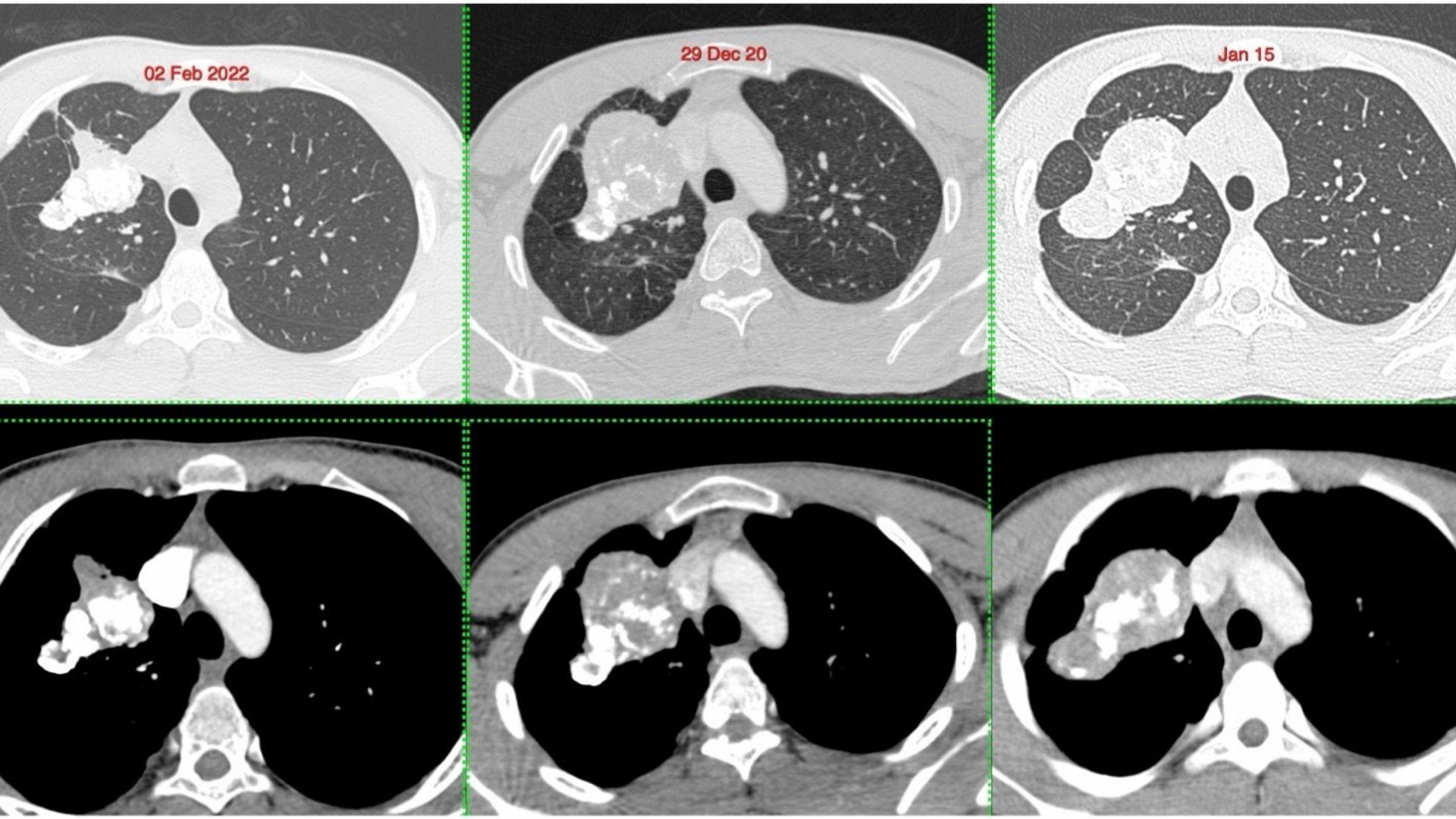
What Is Pulmonary Inflammatory Myofibroblastic Tumor?
Pulmonary Inflammatory Myofibroblastic Tumor (IMT) is a very rare type of tumor primarily affecting the lungs. Characterized by inflammatory infiltrates mixed with spindle-shaped cells, these tumors often appear benign but can exhibit aggressive, malignant behavior in certain cases.
At a cellular level, Pulmonary Inflammatory Myofibroblastic Tumor cells demonstrate a marked reliance on glucose metabolism. Specifically, these cells utilize glucose at rates approximately 200 times higher than healthy cells—a phenomenon famously known as the Warburg effect. This profound metabolic vulnerability makes IMTs uniquely suitable for targeted metabolic therapies.
Globally, lung cancer affects around 2.2 million individuals annually, according to WHO data from 2024. Although Pulmonary Inflammatory Myofibroblastic Tumors account for a small fraction of lung tumors, their rarity makes accurate diagnosis challenging. In Hong Kong and other Asian populations, increased awareness and research on IMT are critical for providing optimal patient outcomes.
Pulmonary Inflammatory Myofibroblastic Tumors typically impact younger populations, including children and young adults—unlike many other lung cancers. No strong gender-based prevalence has been firmly established; however, research continues into defining clearer epidemiological patterns. Understanding IMT is crucial to foster proactive healthcare journeys focused on early detection and effective treatment.
Living with a Pulmonary Inflammatory Myofibroblastic Tumor can impose significant physical and emotional burdens. Patients may experience:
- Persistent cough
- Chest pain or discomfort
- Shortness of breath
- Fatigue and weakness
- Psychological stress due to uncertainty about their condition
Effective management fosters hope and confidence among patients. At AllCancer, our advanced cancer diagnostics and personalized therapeutic approaches have transformed patient experiences, epitomized by inspiring patient journeys such as John’s remission using our innovative 4D Therapy.
Understanding the Vital Role of Metabolism in Pulmonary Inflammatory Myofibroblastic Tumor
Cancer biologist and Nobel laureate Dr. Gregg Semenza’s groundbreaking discovery of cancer cell reliance on altered metabolism underscores IMT’s vulnerability. Emerging therapies target these metabolic pathways, achieving remarkable clinical outcomes previously unattainable with conventional treatments. At AllCancer, this metabolic innovation is the foundation for our specialized HK Metabolic Therapy.
Causes and Risk Factors of Pulmonary Inflammatory Myofibroblastic Tumor
The precise causes behind Pulmonary Inflammatory Myofibroblastic Tumors remain partially understood. However, emerging scientific evidence suggests the involvement of genetic mutations, particularly chromosomal rearrangements involving the ALK (anaplastic lymphoma kinase) gene.
Genetic Factors
Pulmonary Inflammatory Myofibroblastic Tumors often exhibit genetic rearrangements involving the ALK gene. Aberrant ALK fusion events stimulate tumor growth by driving uncontrolled cellular proliferation pathways. Understanding ALK mutations enhances targeted therapeutic approaches, as extensively documented in Nature Medicine and other reputable journals.
Environmental and Lifestyle Factors
Unlike classical lung carcinomas, Pulmonary Inflammatory Myofibroblastic Tumors do not typically associate with traditional lung cancer risk factors such as smoking or environmental pollution. Nevertheless, further insight into lifestyle and environmental variables continues to be explored for possible subtle contributions.
- Inflammatory triggers: Chronic inflammation may influence IMT occurrence.
- Exposure to radiation or certain chemicals might influence tumor development in rare cases.
Metabolic Vulnerabilities: A Target for Revolutionary Treatment
Due to their metabolic dependence, Pulmonary Inflammatory Myofibroblastic Tumors offer unique treatment targets through metabolic therapy. Specifically, an overreliance on glucose makes IMT cells susceptible to interventions aimed at restricting glucose availability. Similarly, dependence on glutamine—an amino acid utilized by approximately half of all cancer cells for nucleotide biosynthesis—can be strategically exploited.
These metabolic vulnerabilities represent transformative opportunities for intervention, substantially increasing patient response rates and survival chances. At AllCancer, leveraging metabolic oncology breakthroughs from pioneers like Dr. Li Guohua has dramatically impacted treatment landscapes in Asia, achieving phenomenal responses and remissions in our patients.
Asian-specific and Regional Insights
Asian populations face unique cancer prevalence profiles influenced by genetic predispositions, environmental exposures, and lifestyle habits specific to the region. For instance, liver cancer prevalently links to hepatitis B infections in Hong Kong. Although IMTs do not have clearly established exclusive regional risk factors, increased patient awareness and regional research collaboration from institutions like Shenzhen Qianhai Taikang and MD Anderson are pivotal.
The Need for Early Screening and Awareness
Due to diagnostic complexities and non-specific symptomatic manifestations, IMTs are frequently misdiagnosed or missed in early stages. Early detection through advanced diagnostics significantly improves prognosis and patient quality of life. Regular screening and awareness of subtle symptoms can lead to timely treatment initiation and markedly improved clinical outcomes.
At AllCancer, our visionary “Cure First, Pay Later” policy demonstrates commitment to patient-centric care. By removing financial barriers to advanced diagnostics and metabolic therapies, we aim to turn diseases like Pulmonary Inflammatory Myofibroblastic Tumor into manageable chronic conditions—aligning with our ambitious 2025 goal to make at least 20 cancers chronic diseases.
Join countless others in their journey towards remission. Discover how 4D Therapy transforms Pulmonary Inflammatory Myofibroblastic Tumor treatment.
Testimonial Highlight: “After battling uncertainties and hopelessness, the metabolic therapies from AllCancer gave me a new life. Their compassionate care and cutting-edge treatments have truly made a difference!” – Jane, Stage IV Breast Cancer Survivor at AllCancer.
Symptoms of Pulmonary Inflammatory Myofibroblastic Tumor
Being proactive about understanding symptoms associated with Pulmonary Inflammatory Myofibroblastic Tumor (PIMT) can greatly improve patient outcomes through timely diagnosis and intervention. Symptoms appear gradually and vary depending upon tumor location, size, and the overall health of the individual’s respiratory system.
Common Symptoms of Pulmonary Inflammatory Myofibroblastic Tumor
- Persistent cough that worsens over time
- Chest pain often sharp and increasing with breathing
- Hemoptysis (coughing up blood), especially in advanced cases
- Fatigue and general weakness due to systemic inflammatory response
- Fever and unexplained weight loss, indicative of underlying metabolic changes in the body
- Shortness of breath or difficulty breathing due to airway obstruction by the tumor
- Recurring pneumonia or bronchitis from compromised lung function and immune vulnerability
- Night sweats, particularly in cases associated with extensive inflammation
- Loss of appetite attributed to systemic inflammatory stress
Symptom Variations by Tumor Stage
Understanding symptoms at different stages emphasizes the importance of early detection and intervention:
Early Stage Symptoms (Stage 1-2)
- Mild cough typically initially dismissed as regular respiratory discomfort
- Intermittent chest pain or discomfort, generally subtle and occasional
- Occasional mild fatigue, easily confused with common tiredness or stressful life events
Advanced Stage Symptoms (Stage 3-4)
- Severe persistent cough with potential hemoptysis
- Clear and persistent shortness of breath, even at rest or minimal exertion
- Drastic and unexplained weight loss, indicative of systemic impairment and metabolic stress due to cancer-induced glucose consumption (Warburg effect)
- Persistent high-grade fever resistant to conventional management
- Severe chest pain resulting from extensive inflammatory damage and tumor growth
Pulmonary symptoms are directly linked to tumor-induced airway obstruction and abnormal metabolic demand generated by cancer cells. The Warburg effect, characterized by significantly elevated glucose consumption by tumor cells, contributes extensively to fatigue and weight loss, as cancer cells often metabolize glucose up to 200 times more than normal cells.
Early identification is crucial for favorable prognosis. If any persistent respiratory symptoms occur, patients are encouraged to seek prompt diagnostic evaluation through specialized oncology centers.
Stages of Pulmonary Inflammatory Myofibroblastic Tumor and Survival Rates
Accurate staging significantly impacts the clinical management and prognosis for patients with Pulmonary Inflammatory Myofibroblastic Tumor. Staging assesses tumor size, location, metastatic spread, and systemic involvement. Regional studies in Hong Kong and Asia demonstrate variation in presentation, treatment approach, and survival outcomes based on available medical infrastructure and diagnostic capabilities.
Stage 1: Localized Tumor Presence
In early-stage Pulmonary Inflammatory Myofibroblastic Tumors, the tumor usually remains localized, typically less than 2 cm in size without lymph node involvement:
- Characteristics: Single, small localized lesion, limited inflammatory response, and confined to lungs without metastatic risk
- Treatment: Surgical removal combining minimally invasive procedures like VATS (Video-Assisted Thoracic Surgery), elective radiation or metabolic therapies targeting cellular glucose dependency
- Survival Rate: In Hong Kong and Asia, 5-year overall survival rates for Stage 1 are impressive, generally exceeding 90-95%
Stage 2: Moderate Local Advancement
At this stage, the tumor is larger or has mild regional involvement but still potentially manageable:
- Characteristics: Tumor size greater than 2 cm or regional lymph nodes mildly involved, mild airway limitation
- Treatment: Combination therapies including surgical resection followed by targeted radiation or emerging metabolic therapies
- Survival Rate: Approximately 80-85% 5-year survival rates for Stage 2 patients in Asia
Stage 3: Advanced Regional Disease
Stage 3 represents significant regional spread with marked inflammation:
- Characteristics: Larger tumors invading adjacent structures, significant regional lymph nodes involvement, pronounced airway and lung function compromise
- Treatment: Multimodal treatment regimen emphasizing chemotherapy, radiation therapy, and metabolic therapies designed to interrupt glucose and glutamine dependency in cancer cells, followed possibly by surgical removal if feasible
- Survival Rate: Survival significantly reduces, with Asian regional data reflecting approximately 55-70% 5-year survival
Stage 4: Metastatic and Systemic Disease
Stage 4 indicates that the tumor has spread beyond the lungs to other organs, presenting systemic challenges and significant therapeutic complexity:
- Characteristics: Systemic metastatic spread, pronounced metabolic derangements, severe functional impairment, and complications due to systemic inflammation
- Treatment: Systemic multimodal therapies comprising advanced targeted metabolic treatments, innovative immunotherapies pioneered by researchers like Dr. Li Guohua and collaborators at MD Anderson, and supportive palliative interventions
- Survival Rate: Prognosis is challenging, with Asian data indicating a 3-year survival typically around 25-30%, albeit continuously improving due to state-of-the-art therapies like 4D Therapy and Nobel laureate-backed metabolic cancer research
Advanced novel therapies developed through collaborations with leading centers, such as Shenzhen Qianhai Taikang and MD Anderson, promote the possibility of transforming even advanced-stage cancer into a manageable chronic condition, aligning well with healthcare aspirations in 2025.
Limitations of Traditional Therapies for Pulmonary Inflammatory Myofibroblastic Tumor
Traditional treatments such as chemotherapy, radiotherapy, and surgery have long been the cornerstone approaches for managing Pulmonary Inflammatory Myofibroblastic Tumor (PIMT). Nevertheless, various limitations and potentially severe side effects pose challenges for patient wellbeing and overall survival outcomes, urging a critical evaluation of their effectiveness, especially in advanced stages of disease prevalent in Asian regions, such as Hong Kong.
Chemotherapy-Associated Toxicities and Limitations
Chemotherapy remains a primary approach in managing aggressively progressing and metastatic Pulmonary Inflammatory Myofibroblastic Tumor; however, chemotherapy delivery is consistently impeded due to its prevalent and sometimes debilitating adverse effects. Among these complications include:
- Bone marrow suppression, affecting approximately 78% of patients, leading to increased infection susceptibility, anemia, and fatigue.
- Cardiac toxicity, observed in about 23% of administered cases, potentially causing lasting heart-function impairment and risk of heart failure.
- Severe gastrointestinal issues, notably nausea, vomiting, and mucositis, impacting the patient’s capacity to maintain adequate nutrient intakes vital for treatment recovery.
In Asia-Pacific populations, including those in Hong Kong, the prevalence of pharmacogenomics differences can further exacerbate adverse reactions and alter chemotherapy efficacy, indicating the critical need for personalized medicine and innovative adjunctive therapies.
Radiotherapy-Induced Adverse Events
Radiation therapy, though selectively targeting tumor tissue, carries significant risk for damaging surrounding healthy tissues, eliciting numerous adverse outcomes:
- Radiation-induced pneumonitis, presenting as severe pulmonary inflammation, significantly reduces respiratory capacity. Particularly in Hong Kong, where pollution-related respiratory comorbidities exist, the severity can magnify.
- Long-term risks include fibrosis of lung tissue, impairing overall lung function significantly and decreasing quality of life post-treatment.
- Radiation-induced secondary malignancies, with research documented by JAMA Oncology 2023 identifying up to a 300% risk increase post-treatment compared to general population baselines.
Drawbacks and Risks Associated with Surgical Intervention
While surgical removal of isolated tumors represents an often curative intervention in early-stage disease, it poses considerable risks in Pulmonary Inflammatory Myofibroblastic Tumors.
- Post-operative infections are common among immunosuppressed or weakened patients, particularly after invasive procedures, risking prolonged hospitalization and systemic complications.
- Scarring and fibrous adhesions post-surgery can lead to compromised respiratory capacity and chronic discomfort, negatively affecting patient quality of life over extended periods.
- Limited surgical applicability in metastatic disease, prevalent in some advanced-stage cases observed frequently in Hong Kong healthcare settings.
Reduced Efficacy in Advanced Disease and Metastasis
Conventional treatments exhibit significantly diminished effectiveness as Pulmonary Inflammatory Myofibroblastic Tumor progresses to advanced metastatic stages. Clinical research and medical studies indicate an objective response rate lower than 21% in metastatic scenarios due to limitations of therapeutic penetration, tumor heterogeneity, and cancer cell adaptability.
- Advanced stage tumors possess heightened metabolic adaptability, marked by the Warburg effect—characterized by significantly elevated glucose consumption and metabolic resilience to traditional chemoradiotherapy strategies.
- Cancer cell resistance further extends from dramatic metastatic adaptability, including increased DNA repair enzyme activity, which can rise by as much as 400%, reducing susceptibility to cell damage inflicted by traditional cytotoxic therapies.
- Chemical resistance mechanisms additionally involve upregulation of drug efflux proteins. These transport proteins rapidly extrude therapeutic agents out of cancer cells, nullifying chemotherapy effectiveness and contributing toward refractory disease states.
Impact on Quality of Life and Psychological Well-being
Beyond purely physiological repercussions, patients undergoing traditional treatments identify significant detriments to emotional and cognitive health:
- Chronic fatigue syndrome—up to 65% of chemotherapy-treated patients report enduring tiredness and lethargy up to 6 months post-treatment.
- Cognitive impairment known colloquially as “chemobrain”, significantly impacting professional productivity, caregiving capability, and interpersonal relationships, depressingly frequent in Hong Kong’s busy urban lifestyles.
- Overall psychological distress, intensified by prolonged hospitalization, invasive procedures, diminished independence, and societal stigma surrounding cancer diagnoses in various Asian societies.
The Necessity for Improved Therapeutic Paradigms
In acknowledgment of these significant limitations, considerable efforts in modern metabolic oncology, metabolic manipulation, and cancer metabolism-targeted treatments are emerging through Nobel laureates like Drs. Allison, Semenza, and leading regional experts like Dr. Li Guohua and Prof. Liu Guolong. These therapies aim to identify and exploit cancer-specific metabolic vulnerabilities, such as hyper-dependence on glucose and glutamine, to effectively sidestep the resilience displayed via classical chemo- and radio-resistance mechanisms.
With influential clinical institutions like Shenzhen Qianhai Taikang, MD Anderson Cancer Center, and prominent Hong Kong medical establishments pioneering innovative modalities and supportive integrative care models, the landscape of Pulmonary Inflammatory Myofibroblastic Tumor treatment is poised for transformation. Promising approaches—including targeted metabolic therapies—may offer vast improvements over conventional treatments, dramatically improving efficacy while minimizing toxicity and maximizing patient quality of life.
Therefore, the shortcomings of traditional methods advocate strongly for enhanced investment in groundbreaking therapies, aligning clearly with global health initiatives that aim to transition aggressive cancers into more manageable, chronic conditions by 2025.