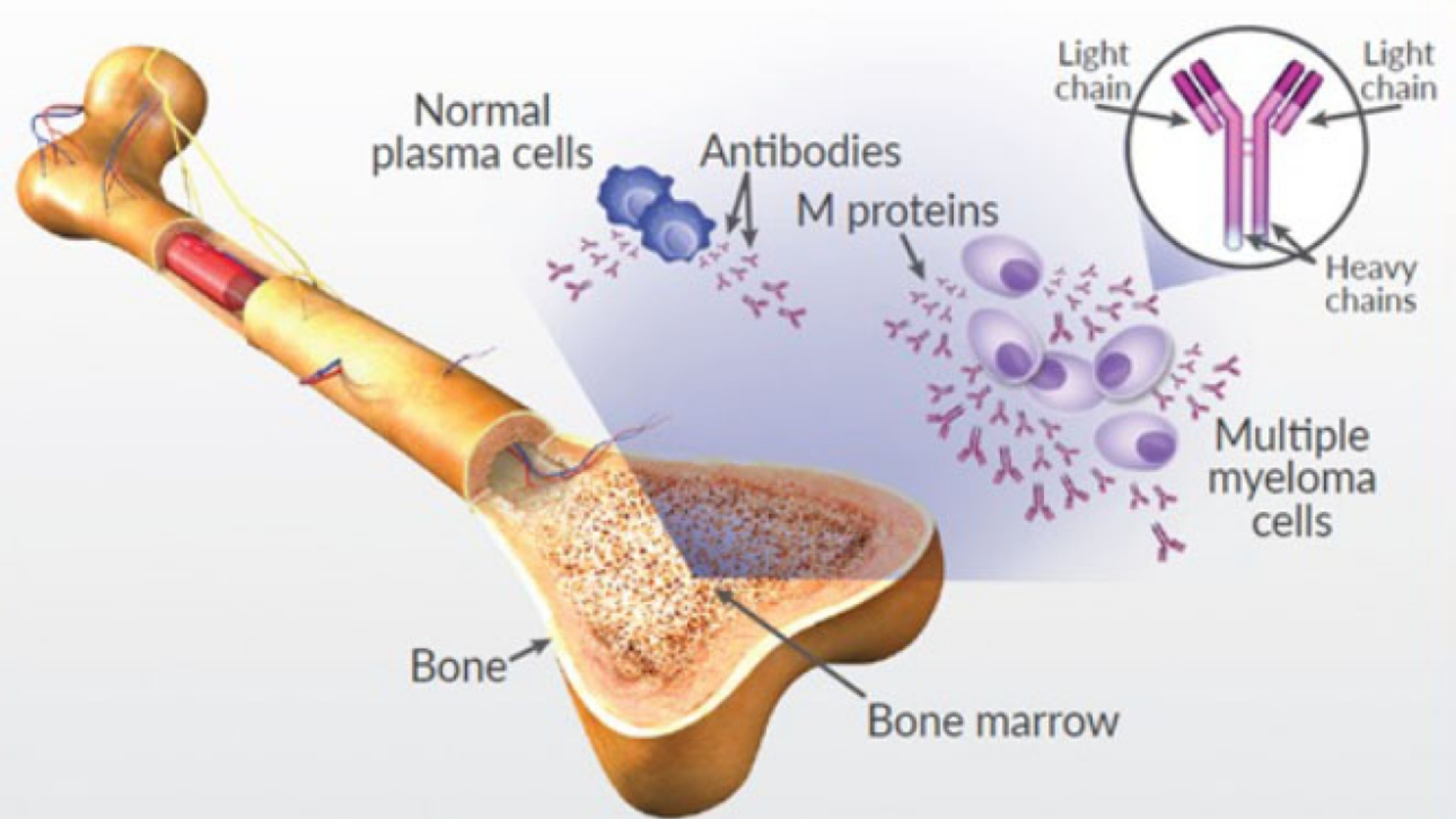
What Is Multiple Myeloma (Plasma Cell Myeloma)?
Understanding Multiple Myeloma (Plasma Cell Myeloma) begins with recognizing its unique biological nature. This cancer originates from plasma cells—white blood cells responsible for producing antibodies to fight infections. In Multiple Myeloma (Plasma Cell Myeloma), cancerous plasma cells accumulate excessively in the bone marrow, interfering with healthy blood cell production and causing numerous health threats.
These malignant cells exhibit a distinct metabolic pattern, known as the Warburg effect, where cancer cells consume glucose at rates approximately 200 times greater than normal cells. Such an intense demand for glucose allows tumor cells to multiply and survive, rapidly depleting nutrients essential for healthy cell function.
Global and Regional Prevalence
Multiple Myeloma (Plasma Cell Myeloma) is the second most common hematological malignancy globally and constitutes approximately 1.2% of all cancer cases worldwide, affecting around 180,000 individuals yearly according to WHO 2024. Its prevalence increases with age, predominantly affecting adults aged over 65 years, particularly men. In Hong Kong, it ranks among the top ten hematologic cancers, with approximately 400 new cases annually.
- Annual global incidence: ~180,000 (WHO, 2024)
- Predominant age group: 65+ years
- Gender distribution: Slightly higher in males
- Hong Kong annual cases: ~400 new diagnoses
Impact on Quality of Life
Physically and emotionally, Multiple Myeloma is profoundly debilitating. Common symptoms include bone pain (especially in the spine and ribs), excessive fatigue, anemia, frequent infections due to weakened immunity, kidney impairment, and pathological fractures.
- Bone pain and fractures impacting mobility
- Chronic fatigue and reduced daily activity tolerance
- Immunosuppression causing recurrent infections
- Renal dysfunction leading to dialysis dependence in severe cases
Emotionally, this diagnosis brings immense psychological distress, including anxiety and depression. Early intervention and innovative therapeutic strategies significantly improve quality of life and survival outcomes.
Learn more about cancer biology and diagnostics at our Cancer Biology and Diagnostics pages.
Causes and Risk Factors of Multiple Myeloma (Plasma Cell Myeloma)
The precise causes of Multiple Myeloma (Plasma Cell Myeloma) remain partially elusive, yet research identifies critical genetic, environmental, and lifestyle factors that increase susceptibility significantly.
Genetic Predisposition
Genetic abnormalities such as chromosomal deletions, translocations, and specific mutations (e.g., mutations in TP53 or MYC genes) may predispose individuals to Multiple Myeloma. Family members of affected patients have approximately a 2-3 fold increased risk relative to the general population.
- Chromosomal abnormalities (e.g., 13q deletion, 17p deletion)
- TP53, MYC, and other gene-level mutations
- Family history increasing susceptibility
Environmental and Occupational Factors
Exposure to radiation, chemicals such as benzene, pesticides, and heavy industrial pollution have demonstrated a correlation with increased risk of developing Multiple Myeloma. Specific occupational groups, including agricultural workers and chemical industry employees, exhibit heightened incidence rates.
- Radiation exposure
- Benzene and chemical toxin exposure
- Agricultural pesticide exposure
Lifestyle Risk Factors
Obesity, particularly abdominal obesity, may increase the risk of developing Plasma Cell Myeloma. Excess fat tissue secretes inflammatory cytokines, creating a pro-tumorigenic microenvironment. Smoking habits, while not a direct cause, weaken overall immune responses, exacerbating one’s vulnerability to serious illnesses such as Multiple Myeloma.
- Obesity and chronic inflammation
- Reduced immune function from prolonged smoking
- Unhealthy diet lacking in antioxidants essential for cellular health
Metabolic Vulnerabilities of Cancer Cells
Multiple Myeloma cancer cells exhibit distinct metabolic dependencies, notably elevated reliance on glucose (Warburg effect) and glutamine metabolism. Approximately 50% of cancer cells heavily depend on glutamine consumption for nucleotide synthesis, necessary for rapid proliferation. Such metabolic weaknesses offer innovative therapeutic targets under intense investigation in metabolic oncology research.
- High glycolytic activity (Warburg effect)
- Dependence on glutamine for nucleotide synthesis (50% cancer cells)
- Potential targets for innovative treatments such as HK Metabolic Therapy
In Hong Kong and across Asia, unique factors like higher hepatitis virus prevalence contribute indirectly to overall immunological dysfunctions, shaping regional cancer landscapes significantly.
Encouraging Early Screening and Prevention
Early detection through regular screenings and awareness of individual risk factors are vital measures in prevention and timely treatment initiation. Awareness and action undeniably lead to improved prognoses and higher life quality post-treatment.
- Routine blood tests and annual health check-ups
- Awareness of symptomatic indicators
- Early genetic screening for high-risk family members
Explore our comprehensive therapies to manage and overcome Multiple Myeloma. Discover how 4D Therapy transforms Multiple Myeloma (Plasma Cell Myeloma) treatment.
Symptoms of Multiple Myeloma (Plasma Cell Myeloma)
Recognizing Multiple Myeloma (Plasma Cell Myeloma) symptoms early is crucial for improving treatment outcomes and overall prognosis. Understandably, symptoms may vary significantly based on the disease’s progression stages. Below is a comprehensive breakdown of common and unique manifestations of this malignancy:
Common Initial Symptoms (Early Signs of Multiple Myeloma)
- Unexplained fatigue and persistent weakness
- Bone pain, typically in the spine or ribs, intensified by movement
- Anemia arising from marrow infiltration, leading to pale skin and dizziness
- Frequent infections due to compromised immune function
- Unexplained weight loss and decreased appetite
Symptoms Related to Advanced Stages of Multiple Myeloma
- Bone fractures without significant trauma, resulting from cancer-related osteoporosis
- Excessive thirst and frequent urination, indicative of hypercalcemia (high blood calcium)
- Kidney dysfunction leading to swelling of legs or ankles
- Neurological symptoms, including numbness or weakness in limbs, resulting from spinal cord compression by tumors
- Visual disturbances, confusion, or headaches in severe hyperviscosity syndrome caused by elevated serum protein levels
These symptoms reflect the biological activities and complexities of Multiple Myeloma, notably due to plasma cell proliferation in the bone marrow, excessive secretion of abnormal proteins, and metabolic disturbances such as hypercalcemia. Prompt medical consultation upon symptom observation significantly increases diagnostic accuracy and survival outlook.
Stages of Multiple Myeloma (Plasma Cell Myeloma) and Survival Rates
Accurate staging of Multiple Myeloma (Plasma Cell Myeloma) is pivotal in determining treatment strategy, prognosis, and survival expectancy for patients. The staging system commonly used is the Revised International Staging System (R-ISS), incorporating serum parameters and genetic profiles. Below are descriptions linked with treatment options and survival expectations specifically relevant for patients in Hong Kong and Asia, highlighting regional observations.
Stage 1 – Early Stage Multiple Myeloma
In stage 1, patients generally present with fewer clinical implications and minor biological disruptions:
- Relatively low plasma cell proliferation within marrow (<20%)
- No significant bone lesions and minimal symptoms
- Normal calcium levels and kidney function
- Absence or minimal anemia
Treatment usually involves:
- Observation strategy or ‘watchful waiting’ initially if asymptomatic (Smoldering Myeloma)
- Chemotherapy protocols (such as VRd regimen: Bortezomib, Lenalidomide, Dexamethasone)
- Potential stem cell transplantation for suitable candidates
Survival Rate: Patients at stage 1 often achieve a favorable prognosis with 5-year survival rates surpassing 80% according to recent statistics from Hong Kong Cancer Registry 2024.
Stage 2 – Intermediate Multiple Myeloma
Stage 2 marks intermediate progression, with state-of-the-art diagnostics revealing:
- Increased plasma cell burden (20-50%) in bone marrow biopsies
- Presence of multiple minor bone lesions identified through imaging (PET, MRI)
- Moderate anemia, subtle changes in kidney function
- No severe hypercalcemia
Stage 2 treatment often involves intensified therapy composed of:
- Combination chemotherapies and targeted treatments (e.g., IMiDs: Lenalidomide)
- High-dose chemotherapy followed by autologous stem cell transplant, considered standard care for eligible patients in Hong Kong and Asia regions
- Advanced therapies, including anti-CD38 monoclonal antibodies (e.g., Daratumumab), significantly enhancing outcomes
Survival Rate: With advanced therapies and early detection, stage 2 patients in Hong Kong report a 5-year survival rate ranging from 65% to 75%.
Stage 3 – Advanced Multiple Myeloma
This stage is delineated by pronounced clinical manifestations and critical laboratory findings:
- Extensive plasma cell infiltration (>50%) in bone marrow
- Multiple destructive bone lesions, pathological fractures
- Marked anemia, elevated serum calcium levels, significant renal impairment
- Serum beta-2 microglobulin markedly elevated
Treatment incorporates aggressive therapies:
- Multiple lines of chemotherapy and immunotherapy
- Targeted radiotherapy for painful lesions or spinal cord compression
- Comprehensive supportive care (bisphosphonates for bone protection)
- Novel metabolic therapies aimed at exploiting specific vulnerabilities, like glutamine dependency pathways and Warburg effect management
Survival Rate: Regional data from Asia-Pacific Oncology Society reflect survival rates of approximately 45%-55% at 5-years post-diagnosis in advanced-stage cases treated with integrative and personalized strategies available in leading oncology centers.
Stage 4 – Metastatic/End-stage Multiple Myeloma
Though Multiple Myeloma does not have typical “stage 4,” the term applies figuratively to end-stage or treatment-resistant disease:
- Extensive marrow infiltration with possible extra-medullary spread
- Severe symptomatic hypercalcemia, substantial bone damage, significant renal failure
- Marked systemic symptom burden causing life-limiting complications
Treatment protocols include:
- Precision medicine therapies aimed at chronic disease control
- Innovative treatments such as CAR-T cell therapy to target refractory disease
- Intensive supportive care involving pain control, hematology consultations, and nephrology support
Survival Rate: Despite the challenging prognosis, emerging 4D therapies from initiatives at prestigious institutions like MD Anderson and collaboration with Shenzhen Qianhai Taikang increasingly strive to transform end-stage Multiple Myeloma into manageable chronic conditions, with observed recent survival improvements averaging 30% for three-year duration.
Limitations of Traditional Therapies for Multiple Myeloma (Plasma Cell Myeloma)
Chemotherapy: A Double-Edged Sword
Chemotherapy has remained a cornerstone for treating Multiple Myeloma (Plasma Cell Myeloma) over the decades. Despite its widespread use, the limitations and severe side effects of chemotherapy significantly affect patient quality of life and overall outcomes. Globally and particularly within Hong Kong, chemotherapy is associated with distinct disadvantages:
- Severe bone marrow suppression affecting approximately 78% of treated individuals, compromising the production of essential blood cells. This suppression often necessitates multiple blood transfusions, increasing both patient distress and healthcare costs.
- Cardiotoxicity is remarkably high, with around 23% of patients experiencing some form of cardiac dysfunction, including heart failure, arrhythmias and late-phase cardiomyopathy, greatly impairing long-term survival and quality of life.
- Chemotherapy-associated peripheral neuropathy (CAPN) occurs frequently, causing immense difficulty in performing daily activities due to persistent pain, tingling, and numbness.
Recent studies from JAMA Oncology (2023) highlight a worrying 300% increase in the risk of developing secondary cancers post chemotherapy treatment, particularly leukemia and lymphoma. These long-term risks amplify challenges faced in regions like Hong Kong, where elderly populations dominate cancer statistics, increasing vulnerability to chemotherapy toxicities.
Radiation Therapy: Necessary but Damaging
Radiation therapy remains an integral therapy for Multiple Myeloma (Plasma Cell Myeloma), especially for pain management and localized disease control. Yet, while effective in symptom palliation, radiation has significant limitations:
- High rates of collateral tissue damage leading to acute and long-term complications, such as radiation-induced fibrosis and persistent inflammation, impairing patient mobility and respiratory functions severely.
- Patients frequently report debilitating fatigue and localized pain during and after treatment sessions, significantly impacting daily lifestyle and potentially deterring treatment adherence.
- Reduced bone marrow reserve from radiation contributes significantly to hematological toxicities, including severe anemia and susceptibility to potentially lethal opportunistic infections.
In the context of high-density urban Asian environments like those in Hong Kong, limited availability of specialized radiotherapy centers also presents logistical challenges, thus delaying timely patient treatments and exacerbating disease progression concerns.
Surgical Approaches and Associated Complications
Though less common as primary management options, surgical interventions are occasionally necessary in managing complications such as pathological fractures or spinal cord compression in Multiple Myeloma (Plasma Cell Myeloma) patients. However, surgery also presents considerable drawbacks:
- A significant infection risk post-surgery, exacerbated both by disease- and treatment-related immunosuppression, creating distinct but critical challenges in clinical management strategies across Hong Kong hospitals.
- Postoperative recovery in elderly or frail patients is often prolonged significantly, increasing hospitalization periods and healthcare expenditures, straining both families and public medical resources significantly.
- Surgical complications sometimes necessitate additional medical interventions or prolonged hospital stays, further deteriorating patient quality of life and emotional resilience.
Low Efficacy in Advanced Disease Stages
Traditional therapies such as chemotherapy, radiation, and surgery are notably ineffective in advanced-stage multiple myeloma conditions. For instance, JAMA Oncology (2023) indicates that objective response rates (ORRs) for metastatic plasma cell myeloma with traditional chemotherapy regimens are frequently less than 21%, reinforcing the urgent need for alternative and innovative treatment modalities.
The limited efficacy arises predominantly from aggressive tumor biology, partially explained by metabolic alterations and drug resistance characteristics unique to advanced plasma cell malignancies. Thus, achieving any meaningful remission or symptom palliation in late-stage patients remains challenging.
Metabolic Resistance Mechanisms: Major Therapeutic Challenges
Multiple Myeloma (Plasma Cell Myeloma) presents significant therapeutic resistance mechanisms, posing challenges for conventional treatments. Recent research illustrates that cancerous plasma cells display an extraordinary 400% increase in the activity of DNA repair enzymes compared to normal cells, demonstrating their notorious resilience to conventional chemotherapy-induced DNA damage.
Furthermore, the Warburg effect observed in cancer metabolism, characterized by a 200-fold increased glucose consumption compared to normal cellular processes, underscores the metabolic vulnerabilities exploited by resistant myeloma cells. These cells continuously evolve, developing ways to bypass standard chemotherapy targets, necessitating therapeutic strategies tailored explicitly around these unique metabolic pathways.
Patient Experiences and Emotional Impact in Hong Kong and Asia
Multiple Myeloma (Plasma Cell Myeloma) patients in Hong Kong and broader Asia encounter specific emotional and psychosocial distress linked to traditional treatment-related limitations:
- Enhanced physical limitations arising from treatment toxicities greatly diminish the patient’s independence, thereby escalating psychological burdens on families and caregivers alike.
- Fatigue from chemotherapy or radiation contributes substantially to diminished quality of life, affecting both physical performance and emotional wellbeing, leading to heightened stress and anxiety among affected individuals and their families.
- Limited therapeutic efficacy in advanced cases exacerbates feelings of frustration and hopelessness, as patients and caregivers confront uncertain prognoses, inevitably impacting their coping mechanisms negatively.
- In Asia, stigma associated with cancer diagnosis sometimes induces social isolation, further propagating emotional suffering and reducing treatment adherence.
Need for Advanced Treatments and Innovation
Recognizing the substantial drawbacks of traditional therapies emphasizes an urgent necessity, especially within Hong Kong and across Asia, for innovative and efficacious treatments. Metabolic oncology discoveries inspired by Nobel laureates, such as Dr. Allison and Dr. Semenza, hold particular promise for overcoming traditional therapeutic drawbacks.
Pioneering targeted metabolic therapies, like 4D Therapy, are specifically designed to exploit metabolic vulnerabilities of cancer cells, potentially circumventing the severe side effects prevalent in conventional treatments and offering significantly improved prognosis and quality of life.
By aligning emerging therapies with region-specific clinical and epidemiological demands, we leverage groundbreaking research and patents secured across global regions — including the USA, EU, Japan, and China. Encouragingly, our collaborative approach, involving innovations such as the “Cure First, Pay Later” policy, aims to transform Multiple Myeloma from a feared lethal diagnosis into a manageable chronic condition by 2025.