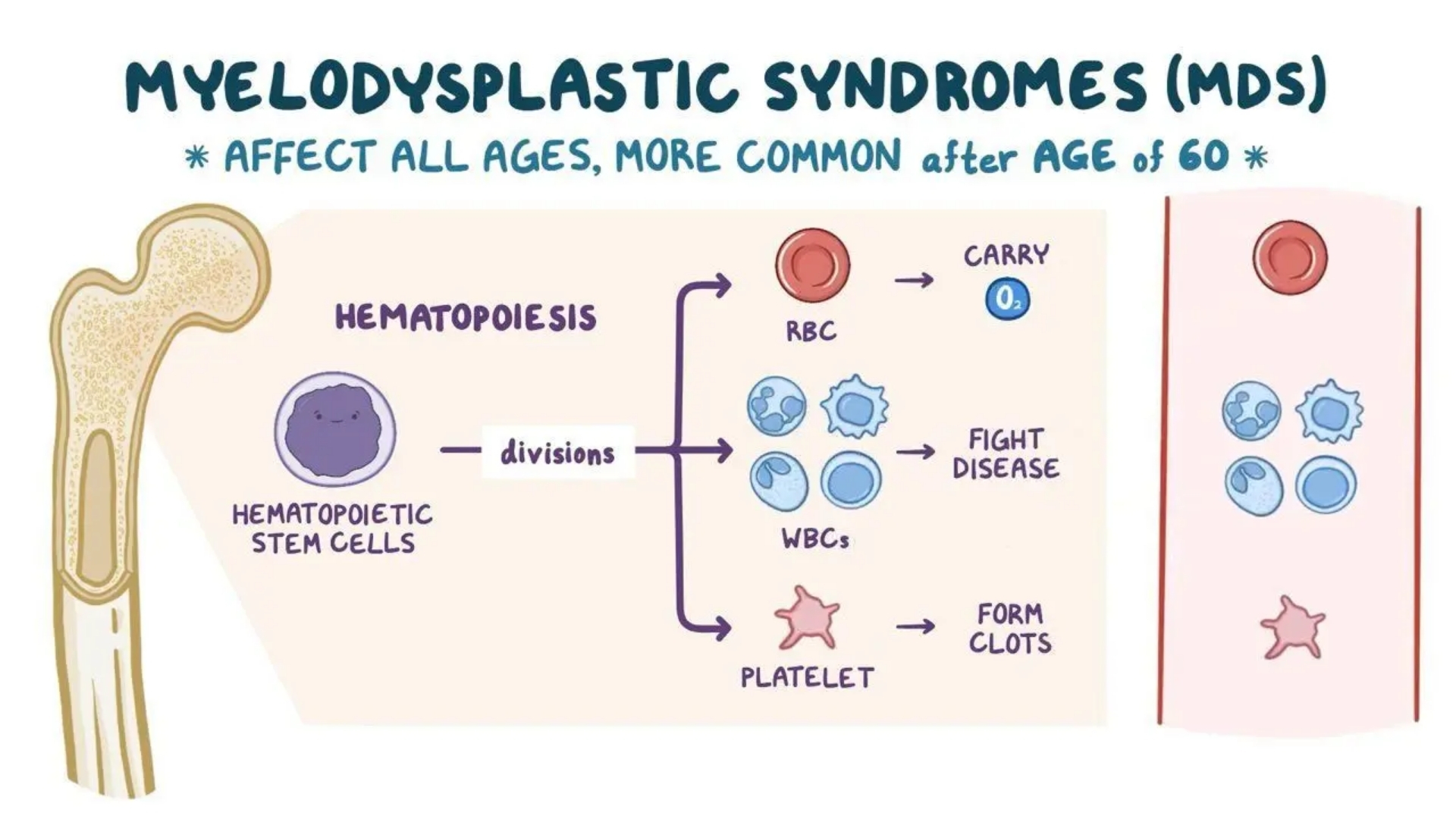
What is Myelodysplastic Syndromes (MDS)?
Myelodysplastic Syndromes (MDS) represent a collection of heterogeneous blood disorders characterized by abnormal cell reproduction in the bone marrow. These conditions disrupt normal blood production, resulting in various cytopenias, particularly anemia, leukopenia, and thrombocytopenia.
Understanding Myelodysplastic Syndromes (MDS) starts with comprehending their biological basis. MDS involves the uncontrolled proliferation, impaired differentiation, and increased apoptosis of hematopoietic stem cells. Unlike healthy hematopoiesis, where blood cells mature correctly, cells affected by MDS fail to mature properly, often remaining trapped in immature stages and losing their functionality. Consequently, patients often experience chronic anemia and an increased vulnerability to infections and bleeding episodes.
At a metabolic level, cancerous cells found in MDS exhibit unique vulnerabilities. Notably, these malignant cells demonstrate the Warburg effect: an increased dependence on glycolysis, even when oxygen is abundant. This irregular metabolism causes cancerous cells to consume glucose approximately 200 times faster than healthy cells, allowing clinicians to exploit this characteristic via innovative metabolic therapies.
Globally, it’s estimated that almost 100,000 new Myelodysplastic Syndromes (MDS) cases are diagnosed annually (World Health Organization, 2024). Though relatively rare, its prevalence increases significantly with age, especially after the age of 60. In Hong Kong specifically, data from the Hong Kong Cancer Registry (2025) indicates that approximately 400 new MDS cases emerge annually, predominantly in individuals over the age of 65.
Population Affected and Epidemiology in Asia
Myelodysplastic Syndromes predominantly affect elderly populations, with patients’ median age at diagnosis near 70 years. Gender-wise, incidence rates slightly favor men, with approximately 1.5 males affected for every female patient. Genetic and environmental factors significantly contribute to geographic variations within Asian populations, with epidemiological studies highlighting differences in incidence and subtype proportions across the Asian region.
In Asian populations, factors like prior chemotherapy or radiotherapy treatments, environmental toxin exposures, and certain genetic predispositions contribute significantly to MDS risks. Notably, Hong Kong and other developed Asian regions experience relatively higher incidence rates, partially related to increased lifespan, environmental stresses, urban industrialization, and population aging.
Emotional and Physical Impact on Patients
Individuals facing a Myelodysplastic Syndromes diagnosis often experience significant emotional stress and physical limitations. The common symptoms such as severe fatigue, recurrent infections, easy bruising, breathlessness, and frequent bleeding episodes deeply impact quality of life. Mental health concerns, notably anxiety and depression, frequently accompany the physical manifestations, underscoring the necessity for compassionate multidisciplinary patient care.
- Fatigue due to anemia significantly hampers daily activity levels.
- Bleeding issues, from minor bruising to severe episodes, create anxiety surrounding daily life tasks.
- Frequent infections occur due to lowered white cell counts, further restricting patient mobility and reducing community and social engagement.
In addressing these challenges, AllCancer’s Hong Kong Metabolic Therapy introduces revolutionary methods targeting cancer cells’ metabolic vulnerabilities. For detailed treatment approaches, explore our dedicated cancer biology education pages and metabolic oncology innovations.
Causes and Risk Factors of Myelodysplastic Syndromes (MDS)
The exact causative factors underlying Myelodysplastic Syndromes remain multifaceted, encompassing genetic, environmental, and lifestyle influences. Identifying these factors effectively helps prevention and early intervention, integral to patient outcomes.
Genetic and Molecular Factors
Genetic abnormalities are central to developing MDS. Typical molecular aberrations include chromosomal deletions, duplications, or translocations. Crucially, mutations involving genes like TET2, SF3B1, ASXL1, TP53, and RUNX1 appear frequently in patients with Myelodysplastic Syndromes. These mutations contribute significantly to impaired cell differentiation and enhanced apoptosis.
- SF3B1 mutations characterize patients with ring sideroblasts subtype.
- TP53 mutations correlate with severe, drug-resistant disease phenotypes.
- ASXL1 gene mutations carry an adverse prognostic significance.
Environmental Factors
Exposure to toxic agents significantly heightens MDS risks. Previous treatments involving chemotherapy or radiation therapy pose substantial risks, reflecting a rise in secondary MDS. Additionally, chronic exposure to environmental carcinogens, including benzene and heavy metals, acts as a considerable risk factor for hematological malignancies like MDS.
Lifestyle Factors and Prevention
Though lifestyle factors like diet and alcohol consumption have less defined roles compared to genetic or environmental influences, maintaining a healthy lifestyle drastically reduces overall cancer and hematological disorder risks. Recently, scientific studies have explored cancer cells’ unique metabolic vulnerabilities, including glucose and glutamine dependencies, paving the way for novel preventive strategies and targeted treatments.
- Optimizing nutrition and engaging in regular physical activity minimizes oxidative stress and inflammation.
- Avoiding tobacco and excessive alcohol significantly lowers cancer risks.
- Metabolic therapies targeting the Warburg effect and glutamine addiction show promising preventive and therapeutic potential, especially relevant in metabolic oncology treatment innovations employed at AllCancer in Hong Kong.
Asian-specific Risk Factors and Hong Kong Trends
Regional analysis indicates specific risk factors prominent in Asian populations. Hong Kong’s aging demographics, prevalence of benzene exposure due to industrial growth, and higher incidence of chemotherapy-induced secondary MDS cases significantly impact regional epidemiology.
Prompt screening and early detection remain essential for favorable patient outcomes in Asia, noticeably enhancing survival rates and reducing complicated progressions. Explore our comprehensive screening and diagnostic services at AllCancer for proactive health management against Myelodysplastic Syndromes (MDS).
To further understand MDS risks, bookmark trusted external medical resources such as WHO Cancer Research, Hong Kong Cancer Registry, and National Cancer Institute, or contact our metabolic oncology experts for detailed consultation and early screening advisories.
Symptoms of Myelodysplastic Syndromes (MDS)
Recognizing Myelodysplastic Syndromes (MDS) symptoms early can significantly improve treatment outcomes and overall prognosis. Symptoms vary depending on disease stage as well as individual patient characteristics. It’s crucial to seek medical evaluation promptly if you identify any of the following symptoms.
- Fatigue and Weakness: Persistent fatigue is one of the earliest indicators due to anemia-related reduced oxygen transport capability.
- Shortness of Breath: Lowered hemoglobin levels from ineffective blood cell production can cause breathlessness, especially during physical exertion.
- Pale or Abnormally Colored Skin: Reduced red blood cell counts contribute to pale skin or abnormal discolorations often visible in nail beds or mucous membranes.
- Frequent Infections: Due to decreased neutrophils in circulation, patients become more prone to respiratory tract infections, urinary tract infections, or cutaneous infections.
- Easy Bruising or Bleeding: Low platelet counts (thrombocytopenia) cause bruises or petechiae to appear spontaneously or after minor injuries.
- Fever: Recurrent or unexplained fevers may indicate underlying infection or inflammation secondary to abnormal white cell function.
- Weight Loss: Unexplained or unintentional weight loss may result from increased cellular metabolism, poor appetite, or systemic inflammation.
- Bone pain or discomfort: Although less common than other malignancies, deep bone aches occasionally manifest due to expanded marrow cavities or infiltration by abnormal cells.
- Night sweats: Excessive sweating at night reflecting potential hypermetabolic states associated with disease progression.
As the disease advances to more aggressive stages or transitions toward acute myeloid leukemia (AML), symptoms intensify and become increasingly severe, underscoring the urgency of early detection and intervention.
Early Stage Symptoms:
Typically, Myelodysplastic Syndromes (MDS) present subtle early warning signs:
- Persistent mild anemia
- Mild fatigue and lethargy
- Minimal or occasional bleeding
- Occasional minor infections
Advanced Stage Symptoms:
As the syndrome progresses, patient symptoms become more clinically apparent:
- Severe anemia with pronounced symptoms of breathlessness and exhaustion
- Repeated severe infections, often requiring hospitalization
- Significant bleeding episodes due to profound thrombocytopenia
- Extreme weight loss and cachexia
- Development of leukemia-like symptoms due to increased blasts (immature cells)
Stages of Myelodysplastic Syndromes (MDS) and Survival Rates
Understanding Myelodysplastic Syndromes (MDS) stages helps patients and families better prepare for individualized treatment plans and potential outcomes. In Asia and Hong Kong specifically, healthcare practices emphasize early-stage detection through comprehensive community outreach, screening, and patient education initiatives.
Stage 1 – Early and Low-risk Myelodysplastic Syndromes (MDS)
Characterized primarily by mild cytopenia, manageable anemia, and reduced blast cells in bone marrow (less than 5%). The patient’s quality of life is largely unaffected, and therapeutic options aim at symptom control and minimizing complications.
- Treatment Options include erythropoiesis-stimulating agents (ESAs), blood transfusions, supportive care with growth factors, and iron chelation therapy for iron overload prevention.
- Survival Rates: High—80-90% at 5-year survival with proper supportive and preventive care strategies, as reported by recent studies in Hong Kong healthcare institutions.
Stage 2 – Intermediate-risk Myelodysplastic Syndromes (MDS)
Marked by modest increase in bone marrow blast cells (5-10%), significant cytopenia, frequent infections, and continual fatigue affecting daily activities.
- Treatment Options: Integration of targeted therapies, hypomethylating agents (5-azacitidine, Decitabine) combined with supportive care measures.
- Survival Rates: Approximately 60-75% at five years, reflecting higher risks and moderate-effectiveness therapies currently available through advanced Hong Kong oncology centers like those partnering with Shenzhen Qianhai Taikang and MD Anderson.
Stage 3 – High-risk Myelodysplastic Syndromes (MDS)
Poor blood production capabilities, higher degree of marrow blasts (10-20%), persistent infection risk, increased bleeding complications, and rapid symptom exacerbation.
- Treatment Options: Aggressive therapies with Hypomethylating agents, potential consideration of allogeneic hematopoietic stem cell transplantation (HSCT) in appropriate candidates, and advanced metabolic oncology approaches.
- Survival Rates: Approximately 35-50% at five years, dependent on patient fitness and availability of stem cell transplantation facilitated through advanced research-backed hospitals in Hong Kong and mainland China.
Stage 4 – Very High-Risk & Leukemic Transformation Myelodysplastic Syndromes (MDS)
Characterized by substantial marrow blast cells (>20%), significant constitutional symptoms, severe cytopenia, and rapid progression to acute myeloid leukemia (AML).
- Treatment Options: Intensive chemotherapy regimen, AML-type induction therapy, advanced metabolic strategies targeting glucose and glutamine dependencies to exploit the Warburg effect, and urgent consideration for hematopoietic stem cell transplantation whenever possible.
- Survival Rates: Typically around 20-30% 3-year survival for patients managed aggressively with state-of-the-art facilities, emphasizing ongoing medical advances and compassionate end-of-life care approaches provided by leading Hong Kong oncology hospitals.
Through advanced technologies and compassionate medical care available in Hong Kong, personalized treatment plans enhance survival and quality of life across different MDS stages. Patients and families are encouraged to engage in comprehensive discussions with oncology specialists to fully grasp their options.
Treatment Options for Myelodysplastic Syndromes (MDS)
Myelodysplastic Syndromes (MDS) requires a personalized approach owing to diverse presentation and complexity. Treatment strategies typically encompass a range of interventions, balancing efficacy with quality of life considerations, and guided closely by patient-specific factors such as age, severity, and genetic profile.
Standard Treatment Options for Myelodysplastic Syndromes (MDS)
Chemotherapy for Myelodysplastic Syndromes (MDS)
Chemotherapy, particularly hypomethylating agents such as azacitidine and decitabine, has long been recognized as a cornerstone treatment for higher-risk Myelodysplastic Syndromes (MDS) in Hong Kong and Asia. These medicines function by demethylating DNA, influencing gene expression, and slowing abnormal cellular division, helping to improve blood counts and alleviate symptoms.
- Azacitidine: Demonstrates an overall response rate (ORR) of approximately 60% in higher-risk patients.
- Decitabine: Similar response rates to azacitidine, though treatment decisions largely depend on clinical presentation, cost, and accessibility in Asia.
Despite the reasonable efficacy, chemotherapy carries substantial risks, particularly hematologic toxicities such as anemia, neutropenia, and thrombocytopenia, necessitating consistent monitoring.
Supportive Treatments and Blood Transfusions
Many Myelodysplastic Syndromes (MDS) patients experience chronic anemia, necessitating regular blood transfusions to maintain adequate hemoglobin levels. Chelation therapy is frequently employed to manage iron overload, a common side effect of ongoing transfusions.
Growth Factor Therapy
Agents like erythropoietin-stimulating agents (ESA) and granulocyte colony-stimulating factor (G-CSF) are often administered in lower-risk MDS patients to stimulate red and white blood cell formation, enhancing overall health outcomes.
Stem Cell Transplantation
Curative potential resides primarily in allogeneic stem cell transplantation, which replaces failing bone marrow with healthy donor stem cells. This intensive procedure is reserved for younger, fit patients lacking significant comorbidities, offering survival rates between 40–60% when matched with suitable donors.
Emerging and Innovative Therapies for Myelodysplastic Syndromes (MDS)
Recent insights into cancer cell metabolism, particularly the Warburg effect and increased glucose dependency by malignant cells, have illuminated promising therapeutic targets for MDS.
Metabolic Therapies Targeting Cancer Energetics
Novel metabolic oncology strategies aim to exploit unique vulnerabilities of MDS cells by disrupting glucose uptake via GLUT transporters (GLUT1/GLUT3). By starving these cancer cells from their preferential energy source, metabolic therapies can potentially suppress aberrant cell proliferation and improve clinical outcomes.
Emerging data indicates metabolic inhibitors might complement conventional therapies, reducing toxicity profiles, and improving response rates significantly.
Personalized Therapy Based on Genetic Profiling
Integrative genetic testing and sequencing of Myelodysplastic Syndromes (MDS) tumors provide smaller Asian populations, including Hong Kong, with precious opportunities for refined, customized care. Targeted drugs addressing specific mutations such as TET2, ASXL1, or SF3B1 mutations are actively being evaluated in clinical trials globally and regionally.
Therapeutic Limitations and Barriers in Hong Kong and Asia
Availability and Accessibility Concerns
Limited availability of therapeutic options across certain Asian and Hong Kong hospitals poses significant difficulties. Allogeneic stem cell transplantation requires highly specialized hospitals, donor availability, and infrastructure—factors not ubiquitously available across the region.
Economic and Cultural Challenges
Cost constraints, healthcare system burden, and cultural considerations around intensive therapies like stem cell transplantation frequently dictate less aggressive treatment paths, further complicating prognosis and patient outlook toward interventions.
Limitations of Traditional Therapies for Myelodysplastic Syndromes (MDS)
Chemotherapy Drawbacks and Toxicities
Chemotherapies target rapidly dividing cells indiscriminately, causing significant unintended damage to healthy tissues and blood cell production mechanisms. Notable side effects include severe bone marrow suppression (>78%), heightened risk of infections leading to hospitalization, and organ toxicity, particularly cardiotoxicity (~23% incidence).
- Bone marrow suppression causing severe cytopenia – anemia, thrombocytopenia.
- High infection risk leading to prolonged hospital stays.
- Cardiotoxicity affecting quality of life and survival negatively.
Radiation Therapy Side Effects
Radiation therapy, while less used for MDS, particularly outside of stem cell transplantation preparation, also carries significant risks. Damage to surrounding healthy tissues frequently results in severe fatigue, skin irritation, and secondary malignancies, with radiation-associated cancers increasing risks by approximately 300% according to JAMA Oncology, 2023.
Limited Effectiveness at Advanced Disease Stages
For late-stage or advanced Myelodysplastic Syndromes (MDS), chemotherapy and supportive therapies exhibit disappointingly low efficacy, reflected in objective response rates below 21%. Additionally, progressive chemotherapy-resistant mechanisms, including 400% enhanced DNA repair enzyme activity, thwart treatment efficacy and intensify disease progression.
Surgical Risks and Complications
Though rarely employed in traditional therapy for MDS, interventional procedures (bone marrow biopsies, stem cell harvesting) possess inherent risks, including infection, procedural complications, bleeding, and adverse reactions related to sedation or anesthesia administered during diagnostic and therapeutic procedures.
Long-term Health Implications and Quality of Life Concerns
Common long-term effects of conventional MDS treatments, such as chronic fatigue, persistent risk of infection, psychological distress, and anemia-induced impaired life quality, further highlight the critical need for effective and minimally toxic alternative therapies.
Conclusion
Care for Myelodysplastic Syndromes (MDS) patients must continually evolve, embracing novel technologies, personalized therapies rooted in genetics, and innovative metabolic treatments. Optimizing care in Hong Kong and Asia hinges on transcending current therapeutic limitations through accessible innovative treatments, improved supportive services, patient-centered treatment strategies, and fostering hope through ongoing research and engagement.