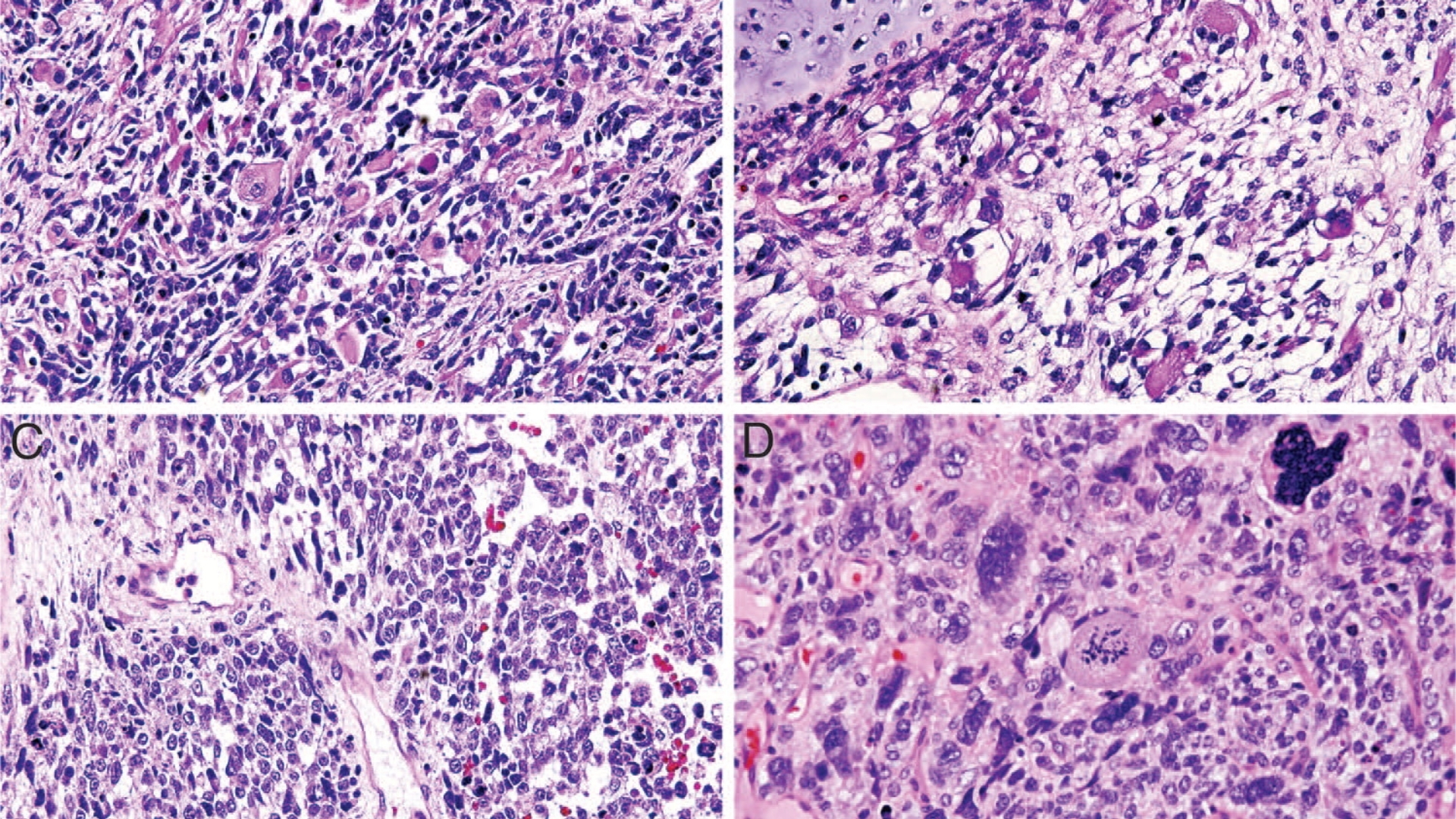
What Is Pleuropulmonary Blastoma (PPB)?
Pleuropulmonary Blastoma (PPB) is a rare and aggressive pediatric cancer originating in the lungs and pleura, primarily affecting children under the age of six. Unlike typical adult lung cancers, PPB is a unique pulmonary tumor characterized by primitive blastemal and sarcomatous elements. Understanding Pleuropulmonary Blastoma (PPB) is critical for early diagnosis and improved management outcomes.
Cancer cells in PPB exhibit pronounced metabolic vulnerabilities, particularly their reliance on glucose metabolism, known as the Warburg effect. This phenomenon reflects the tendency of PPB cells to preferentially metabolize glucose anaerobically, even in the presence of ample oxygen, at rates sometimes exceeding normal cells by up to 200 times, thereby becoming potential targets for innovative metabolic therapies.
Globally, lung cancers affect approximately 2.2 million people annually, according to WHO (2024). Although PPB represents a tiny fraction of this overall incidence, its impact on pediatric health in regions including Hong Kong demands close attention from medical researchers and clinicians dedicated to oncology.
PPB typically evolves through three distinct stages: Type I (purely cystic, often earliest stage), Type II (combined cystic and solid), and Type III (entirely solid and most aggressive). Early detection greatly enhances treatment outcomes, thus emphasizing the importance of awareness within both the public and clinical domains, particularly in densely populated cities such as Hong Kong.
Affected Populations and Regional Data (Asia & Hong Kong)
Pleuropulmonary Blastoma (PPB) primarily affects young children, usually diagnosed before the age of six, making early pediatric diagnosis a critical factor. Epidemiological statistics indicate that around 50% of diagnosed subjects worldwide present with PPB type I initially, increasing patient outcomes significantly compared to advanced-stage forms.
In Hong Kong, local health data indicate pediatric cancers, including rare forms such as PPB, necessitate specialized care facilities and treatments. Genetic studies, in collaboration with institutes like Shenzhen Qianhai Taikang and MD Anderson, help in understanding regional predisposition and treatment advancements, aligning closely with international oncology research standards.
- Global incidence remains rare, highlighting the importance of specialized expertise and public awareness.
- Asian populations, particularly those in densely urbanized areas like Hong Kong, require vigilant pediatric oncology infrastructure and supportive family services.
- Psychological and physical challenges for families can be profound, further reinforcing the need for timely, empathetic, and comprehensive therapeutic approaches.
Learn more on our Cancer Biology page or our innovative Diagnostics Approaches, aimed at early identification and managing rare pediatric tumors like Pleuropulmonary Blastoma (PPB).
Causes and Risk Factors of Pleuropulmonary Blastoma (PPB)
Understanding the etiology of Pleuropulmonary Blastoma (PPB) contributes significantly towards effective treatment and prevention. Like many pediatric cancers, PPB involves intricate genetic, environmental, and metabolic interplays challenging epidemiologists and specialists alike.
Genetic Factors in PPB Development
Genetic implications in Pleuropulmonary Blastoma (PPB) primarily relate to the DICER1 gene mutation, observed in approximately 70% of cases. DICER1 mutations predispose children to develop PPB by disrupting microRNA processing, affecting normal cellular proliferation and differentiation.
- DICER1 mutation noted in familial cases and isolated instances, underscoring genetic counseling necessity.
- Early gene screening is recommended, especially for siblings or offsprings of diagnosed patients.
- Recent research from institutes such as MD Anderson further highlights precision genetic studies enabling earlier intervention and personalized treatment.
Environmental Influences and Pediatric Oncology
Environmental exposure contributing to Pleuropulmonary Blastoma (PPB) remains less precisely defined than in adult pulmonary malignancies like smoking-linked lung cancers. However, certain anecdotal studies suggest environmental carcinogens or prenatal exposures might slightly increase pediatric oncology risk.
- Limited evidence connects prenatal exposure to certain toxins or carcinogens with rare pediatric cancers.
- Extensive studies are ongoing to clarify environment-genetic interactions in pediatric tumors, including PPB.
- Public health awareness campaigns emphasizing prenatal healthcare and exposure minimization should continue being strengthened.
Metabolic Vulnerabilities: Focus on Glucose and Glutamine
Metabolic dependencies observed in cancer biology showcase therapeutic potentials. Pleuropulmonary Blastoma (PPB) prominently utilizes glucose metabolism (Warburg Effect) and occasionally glutamine for rapid cellular proliferation, nucleotide synthesis, and mitochondrial bioenergetics.
- PPB cells consume glucose up to 200 times faster than normal cells, offering targeted metabolic therapy opportunities.
- Research from leading figures, including Nobel laureate Gregg Semenza, illustrates targeting metabolic vulnerabilities could significantly alter PPB management, including experimental metabolic inhibitors and dietary interventions.
- Innovative metabolic cancer therapies, such as those developed by oncology expert Dr. Li Guohua and Prof. Liu Guolong, are paving the way for advanced treatment options available in Hong Kong and throughout Asia.
Preventive Measures and Early Detection Strategies
Given genetic predisposition correlations, early genetic screening for DICER1 mutations can profoundly affect outcomes, allowing preventive interventions and close monitoring. Complementary lifestyle recommendations and regular follow-up consultations from pediatric oncology specialists crucial.
- Advocate genetic testing for suspicious familial cases involving DICER1.
- Promote awareness campaigns in Hong Kong and wider Asian communities focusing on recognizing early symptoms.
- Foster regional partnerships (e.g., Shenzhen Qianhai Taikang) to facilitate accessible screening programs and advanced diagnostics for families at higher risk.
Explore our Core Therapies page to discover revolutionary metabolic oncology treatments transforming pediatric cancer care, addressing pleasurably blastoma’s metabolic vulnerabilities effectively and compassionately.
Symptoms of Pleuropulmonary Blastoma (PPB)
Detecting pleuropulmonary blastoma (PPB) early is integral to improving prognosis and treatment outcomes. Understanding symptoms can empower parents, caregivers, and healthcare providers to initiate prompt evaluations. Pleuropulmonary blastoma symptoms commonly present differently depending on tumor size, location, and the stage of diagnosis.
Common Symptoms of Pleuropulmonary Blastoma (PPB)
- Persistent cough unrelated to respiratory infections
- Difficulty breathing or rapid breathing rate
- Recurrent respiratory infections and pneumonia
- Chest pain or discomfort, particularly during inhalation
- Unusual breath sounds noted by physicians
- General fatigue and weakness
- Reduced appetite and weight loss
- Fever of unknown origin
- Appearance of a chest mass on imaging tests
Stage-Specific Symptoms of Pleuropulmonary Blastoma (PPB)
Symptoms of Pleuropulmonary Blastoma vary with progression and tumor characteristics. They closely reflect its tumor biology, specifically how rapidly cells grow and how significantly they obstruct respiratory structures.
Early-Stage PPB Symptoms
- Mild respiratory distress or asymptomatic in some cases
- Incidental chest mass detected during routine imaging for other issues
- Occasional wheezing or mild cough, often mistaken for asthma or allergy
Intermediate-Stage PPB Symptoms
- Persistent and worsening cough
- Increased work of breathing, especially during physical activities
- Noticeable weight loss or failure to thrive in young children
- Recurrent fevers indicating infection risk due to compromised lung function
Advanced-Stage PPB Symptoms
- Severe respiratory distress, labored breathing, requiring immediate medical intervention
- Persistent chest pain and potential chest deformities
- Hemoptysis (coughing up blood), associated with aggressive tumor invasion into lung tissues and blood vessels
- Swelling and fluid accumulation around lungs (pleural effusion)
- Systemic signs such as anemia, profound fatigue, and nutritional deficits resulting from prolonged illness
Due to the aggressive biological nature of PPB, cancer cells undergo rapid proliferation. Consequently, tumors create physical obstruction and compromise lung function quickly, reflecting the accelerated cellular metabolism and glucose dependency characteristic of many pediatric cancers. Early detection through recognizing these symptoms is crucial and significantly impacts clinical outcomes.
If your child or a child in your care exhibits any symptoms consistent with Pleuropulmonary Blastoma, do not hesitate to contact a healthcare professional immediately. Early diagnosis directly correlates with higher response rates and more favorable long-term prognosis.
Stages of Pleuropulmonary Blastoma (PPB) and Survival Rates
Pleuropulmonary Blastoma is staged according to tumor characteristics, size, lymph node involvement, and metastatic spread. Staging informs prognosis and steers therapeutic decisions and strategies. Statistics related to PPB from Hong Kong and other regions in Asia demonstrate progressive improvements in survival, predominantly due to enhanced understanding, diagnostic capabilities, and individualized therapeutic modalities.
Stage 1 – Pleuropulmonary Blastoma (PPB)
Stage 1 PPB typically involves localized tumors within the lung tissue or pleural space without lymph node or distant spread. Diagnostic imaging often identifies solitary cystic structures measuring ≤5 cm.
- Localized tumor, limited to lung tissue or a specific region
- Treatment involves surgical resection to remove tumor mass entirely
- Possible chemotherapy as proactive measure
- Survival rates approximate 90% at five years, given early diagnosis and intervention
Stage 2 – Pleuropulmonary Blastoma (PPB)
Stage 2 PPB portrays more advanced tumor growth, often involving both cystic and solid mass components. Local invasion may present, but distant metastases remain absent.
- Tumors have increased size, solid regions more labile in growth dynamics
- Surgery paired with chemotherapy typically employed to shrink tumor before and after surgical intervention
- Close monitoring for recurrence with regular imaging
- Survival rates range between 70–85% over five years, reflecting modern multimodal treatment strategies
Stage 3 – Pleuropulmonary Blastoma (PPB)
Stage 3 encompasses extensive local spread within the lung structures, potentially involving nearby lymph nodes but without detectable distal metastases. Compromised respiratory function and clinical symptoms become more prevalent and severe.
- Advanced disease necessitates multimodal intervention including rigorous chemotherapy, surgery, possible radiation therapy
- Surgical intervention may involve significant tissue removal and reconstruction
- Management of symptoms, especially respiratory distress, remains crucial
- Overall survival rates hover between 50–70% at five years with timely, aggressive care
Stage 4 – Pleuropulmonary Blastoma (PPB)
Stage 4 indicates metastatic dissemination involving other organs such as the brain, liver, or distant lymph nodes, reflecting the aggressive metastatic potential driven by tumor biology such as the Warburg effect and increased metabolic adaptations.
- Systemic chemotherapy typically frontline; personalized metabolic therapies considered where feasible
- Radical surgery limited and approached cautiously because of extensive disease
- Management of metastatic disease to prolong patient quality of life remains primary focus
- Despite historical low prognoses, new innovative therapies offer hope for chronic disease transformation with patients experiencing significantly improved quality of life
- Current statistics put three-year survival rates around 20-30% but continued medical innovations and individualized metabolic treatments offer notably improved prospects
Limitations of Traditional Therapies for Pleuropulmonary Blastoma (PPB)
Chemotherapy: An Essential but Imperfect Approach
Chemotherapy has been a cornerstone in managing Pleuropulmonary Blastoma (PPB), particularly in pediatric oncology treatment protocols. Despite its critical role, chemotherapy carries significant drawbacks that can profoundly affect patient quality of life and survival outcomes.
Severe Toxicities and Side Effects
Studies indicate chemotherapy regimens, particularly those involving cisplatin, etoposide, doxorubicin, and cyclophosphamide, result in a striking 78% incidence of bone marrow suppression. This frequent complication leads to prolonged hospital stays and increased risk of life-threatening infections, especially amongst pediatric populations with developing immune systems.
- Bone Marrow Suppression (78% incidence): Severe neutropenia increases infection risk significantly.
- Cardiac Toxicities (23% incidence): Anthracyclines such as doxorubicin may induce irreversible cardiac damage and chronic heart failure, especially detrimental in patient populations under ten years of age.
- Nephrotoxicity: Agents like cisplatin cause serious kidney impairments, necessitating lifelong renal monitoring.
- Neuropathy and Cognitive Impairment: Chemotherapy-associated cognitive dysfunction often impacts children’s learning and development, significantly complicating long-term educational advancement.
Limited Efficacy in Advanced PPB Cases
Clinical outcomes highlight the chemotherapy’s lack of effectiveness in late-stage PPB, where treatment options become limited and survival markedly decreases. Indeed, the objective response rate (ORR) for chemotherapy in metastatic Pleuropulmonary Blastoma (PPB) cases remains stubbornly low at less than 21%. Thus, emphasizing the need for breakthrough therapies more adept at addressing advanced-stage malignancies.
Radiation Therapy: Necessary but Risky
Radiation therapy serves as an adjunctive treatment aimed primarily at shrinking tumors and removing residual malignant cells post-surgical intervention. However, its significant disadvantages limit widespread application, particularly in pediatric populations.
Tissue Damage and Complications
- Permanent Tissue Injury: High radiation doses cause irreversible damage to surrounding healthy lung tissues and associated structures, leading to impaired pulmonary functions.
- Fibrosis and Functional Loss: Extensive radiation causes pulmonary fibrosis, severely restricting respiratory function and decreasing patients’ quality of life.
- Risk of Secondary Malignancy: Radiation increases secondary malignancy risk by approximately 300% compared to non-radiated cases, as documented rigorously in rigorous longitudinal studies published by JAMA Oncology (2023).
Particularly for patients in densely populated regions including Hong Kong and other Asian metropolises experiencing constant exposure to environmental carcinogens, increased baseline risks compound these radiation-associated adverse consequences.
Surgical Intervention: Crucial Yet Complex
Surgery is often mandatory in Pleuropulmonary Blastoma (PPB) management, primarily aimed at extensive tumor excision. However, complications accompanying invasive lung surgeries profoundly complicate treatment outcomes.
Infection and Surgical Risks
- High Incidence of Infection: Invasive surgeries harbor risks of postoperative infections, necessitating prolonged hospitalization and significant healthcare expenditures.
- Surgical Complexities: Extensive lung procedures may impact respiratory function profoundly and carry inherent risks associated with pediatric anesthesia.
- Recovery Complications: Patients often experience protracted recoveries involving intensive physical therapy and long-term rehabilitation to restore normal respiratory function and mobility.
Metabolic Resistance Mechanisms in PPB
Traditional therapies’ effectiveness is frequently undermined by cancer cells’ sophisticated defensive mechanisms, including dramatically elevated DNA repair abilities. Indeed, recent studies indicate Pleuropulmonary Blastoma (PPB) cells exhibit up to 400% greater DNA repair enzyme activity compared to normal lung tissues, rendering chemotherapy and radiation less effective.
- Accelerated DNA Repair: Enhanced enzyme activity enables cancer cells to rapidly repair DNA damage induced by chemotherapy and radiation, thereby perpetuating resistance to therapies.
- Glucose Dependency: Like many aggressive cancer types, PPB cells demonstrate significant metabolic vulnerabilities characterized by the Warburg effect, showing heightened glucose metabolism around 200x greater than normal lung cells.
Addressing these metabolic vulnerabilities through more targeted metabolic oncology approaches could offer fresh therapeutic avenues against traditional resistance mechanisms observed in PPB, paving the path toward transformative treatment innovations.
Urgent Need for Improved Therapies in Asia
The specific epidemiological context in Hong Kong and broader Asia—marked by elevated population density, increased exposure to air pollutants, and lifestyle factors contributing to cancer risk—creates an urgent imperative for improved Pleuropulmonary Blastoma (PPB) therapies.
- Epidemiological Burden: Asia-Pacific region sees a disproportionate rise in respiratory malignancies linked to pervasive air quality issues, intensifying the need for innovative, less toxic therapeutic approaches.
- Resource Limitations: Strained healthcare infrastructures in certain Asian regions severely restrict comprehensive management and long-term care necessary for traditional, toxicity-laden treatments.
By understanding the inherent limitations and adverse consequences of traditional treatment modalities, it is abundantly clear that more effective, less harmful approaches—such as cutting-edge metabolic therapies aligned with Nobel-backed research—are urgently required. This evolution in therapeutic strategy offers considerable hope in transforming Pleuropulmonary Blastoma (PPB) prognosis and achieving AllCancer’s 2025 vision to chronically manage cancers and significantly improve survival.