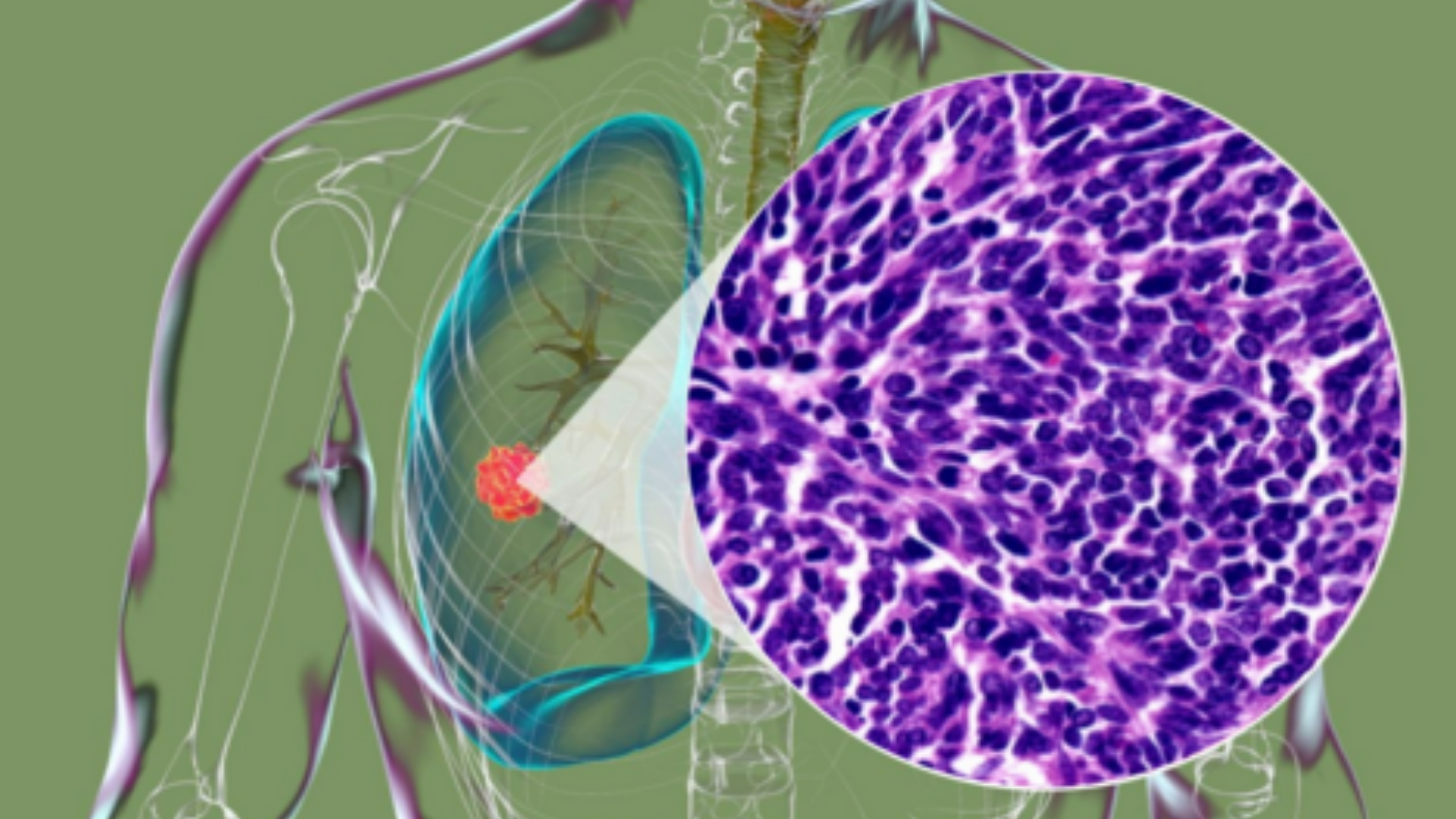
What Is Small Cell Lung Cancer (SCLC)?
Small Cell Lung Cancer (SCLC) is an aggressive form of lung cancer characterized by small-sized tumor cells that multiply rapidly. This cancer originates predominantly within the central bronchi and quickly spreads to lymph nodes and distant organs, making it a severe condition impacting patient survival and quality of life significantly.
Biological Basis of Small Cell Lung Cancer (SCLC)
At the biological level, Small Cell Lung Cancer (SCLC) often demonstrates unique metabolic alterations, notably the Warburg effect. This phenomenon describes cancer cells fermenting glucose anaerobically even in the presence of oxygen, consuming glucose at nearly 200 times the rate of normal cells, resulting in rapid tumor growth and proliferation.
- Rapid glucose metabolism (Warburg effect)
- High dependency on glycolysis and glutamine metabolism
- Rapidly proliferating cancer cells
Prevalence and Epidemiology
Globally, lung cancer impacts approximately 2.2 million people annually, according to WHO data from 2024. Small Cell Lung Cancer specifically represents about 15% of all lung cancer diagnoses but holds significant morbidity due to rapid progression and early metastasis.
In Hong Kong and East Asia, the prevalence of lung cancer remains high due to regional-specific factors, notably high tobacco consumption rates, air pollution, and genetic predispositions such as EGFR gene mutations. Traditionally more common among males aged 55–70, recent trends show an increasing incidence among females and younger demographics.
- Annual incidence over 5,000 new cases in Hong Kong (2024)
- Shift toward younger age group diagnosis
- Females increasingly affected due to changes in smoking patterns
Impact on Patients: Emotional and Physical Burden
A Small Cell Lung Cancer diagnosis carries intense emotional and physical burdens. Patients typically experience symptoms including persistent coughing, bloody sputum, chest pain, fatigue, weight loss, and respiratory distress. The aggressive nature of SCLC exacerbates emotional distress, anxiety, and feelings of isolation, emphasizing the importance of compassionate and timely care.
- Pain and respiratory difficulties
- Weight loss and fatigue
- Heightened psychological distress and anxiety
Causes and Risk Factors of Small Cell Lung Cancer (SCLC)
Understanding Small Cell Lung Cancer (SCLC) entails discussing certain critical risk factors and causes. These encompass genetic mutations, lifestyle habits, environmental exposures, and unique metabolic vulnerabilities of cancer cells that significantly contribute to the onset of the disease.
Genetic and Molecular Factors
Genetic alterations, particularly mutations in tumor suppressor genes such as TP53 and RB1, are commonly associated with Small Cell Lung Cancer (SCLC). These genetic aberrations reflect a vulnerability that can potentially be targeted in modern therapeutic approaches.
- TP53 and RB1 mutations occurring in ~90% of SCLC patients
- Additional involvement of PIK3CA, PTEN, and EGFR mutations in subsets of patients
- Ongoing research-driven genetic profiling for personalized treatments
Lifestyle Factors and Environmental Influences
Smoking remains the predominant risk factor, responsible for over 80% of Small Cell Lung Cancer cases. Environmental exposure to air pollutants, asbestos, radiation, and secondhand smoking are additional considerable contributors, particularly in densely populated urban centers like Hong Kong.
- Tobacco smoking (including cigarette, cigar, pipe, and secondhand smoke)
- Exposure to asbestos, radon, and industrial pollutants
- Chronic exposure to high levels of urban air pollution
Metabolic Dependencies in Cancer Cells
Cancer cells exhibit high metabolic flexibility yet commonly rely on specific metabolic pathways such as glucose and glutamine metabolism. Approximately 50% of cancer cells, including SCLC cells, exploit glutamine heavily for nucleotide synthesis, signaling pathways, and maintaining proliferative capacity.
- High reliance on glucose fermentation (Warburg effect)
- Dependence on glutamine for cell proliferation and survival
- Potential therapeutic targeting with metabolic inhibitors (e.g., GLS inhibitors)
Specific Risks in Hong Kong and Asia
In Asia and particularly Hong Kong, distinctive regional risk patterns emerge. Elevated smoking rates, widespread urban pollution, dietary lifestyles, and genetic susceptibility (such as frequent EGFR mutation prevalence noted in East Asian populations) create a unique environment leading to noticeable risk clustering in this region.
- EGFR mutations prevalent in Asian populations (approximately 40–50% of patient cases)
- Increased urban pollution due to high population density
- Dietary influences and lifestyle-driven factors (high-fat diets and lack of physical activity)
Preventive and early detection measures
Promoting early screenings, such as regular chest X-rays, low-dose CT scans for high-risk individuals, and genetic counseling for susceptible populations, significantly improves outcomes for SCLC patients. Educating the public about modifiable risk factors (such as smoking cessation, lifestyle improvements, and environmental intervention) remains imperative.
- Regular screenings and early diagnostic tests
- Lifestyle modifications (smoking cessation, healthier dietary practices)
- Precision medicine and personalized management
Discover how 4D Therapy transforms Small Cell Lung Cancer (SCLC) treatment
Explore the stories of hope and recovery, including John’s lung cancer remission with 4D Therapy, as we pursue our goal to make Small Cell Lung Cancer (SCLC) a chronic and manageable disease by 2025.
Symptoms of Small Cell Lung Cancer (SCLC)
Recognizing the symptoms of Small Cell Lung Cancer (SCLC) is critical for early detection, improving the prognosis, and timely intervention. Symptoms tend to vary based on stage and can dramatically impact the patient’s quality of life. Familiarizing yourself with these indicators is necessary for early diagnosis.
Common Early Stage Symptoms of Small Cell Lung Cancer (SCLC)
- Persistent cough that does not resolve with standard cold or flu treatments
- Hoarseness lasting over two weeks
- Chest discomfort or dull aching pain
- Episodes of hemoptysis (coughing up blood)
- Unexplained fatigue that significantly limits daily activities
- Increasingly frequent episodes of lung infections such as bronchitis or pneumonia
Symptoms Reflecting Tumor Biology
Small Cell Lung Cancer (SCLC) has unique biological characteristics driven by rapidly proliferating malignant cells. Understanding tumor biology helps us correlate symptoms with specific pathological mechanisms:
- The persistent cough is primarily due to airway irritation and obstruction caused by the tumor.
- Hemoptysis occurs as the tumor invades delicate blood vessels within the bronchial walls.
- Hoarseness is related to recurrent laryngeal nerve invasion, a sign that warrants immediate evaluation.
- Frequent lung infections signal the compromised immune response and airway blockage from tumor growth.
Advanced-Stage Symptoms of Small Cell Lung Cancer (SCLC)
- Severe bone pain due to metastases, common in late-stage disease
- Neurological manifestations such as headaches, dizziness, or seizures from brain metastasis
- Unexplained weight loss and anorexia caused by cancer-induced metabolic changes
- Painful swelling and coughing due to malignant pleural effusions (fluid accumulation surrounding the lungs)
- Difficulty swallowing from esophageal compression or invasion
- Weakness or numbness typically resulting from spinal cord compression (associated with spinal metastasis)
Early evaluation and diagnosis by medical specialists can dramatically improve outcomes. If you experience any persistent respiratory symptoms, please promptly consult a healthcare provider for appropriate investigations and personalized therapy plans.
Stages of Small Cell Lung Cancer (SCLC) and Survival Rates
The staging of Small Cell Lung Cancer (SCLC) plays a pivotal role in determining prognosis and selecting the most effective therapies. Only with accurate stage determination can patients achieve optimal outcomes. Survival rates vary significantly among stages and directly influence treatment strategies.
Stage 1 – Small Cell Lung Cancer (SCLC)
Stage 1 is characterized by localized tumors strictly confined within one lung, typically smaller than 3 centimeters with no lymph node involvement. It accounts for approximately 5–10% of diagnosed SCLC in Hong Kong and Asia.
- Treatment Options:
- Surgery is potentially curative for eligible patients.
- Radiotherapy can significantly enhance surgical or standalone outcomes.
- Early chemotherapy regimens help decrease recurrence significantly.
- Survival Rates:
- Patients diagnosed at Stage 1 have a promising prognosis of 50–60% five-year survival with comprehensive therapy intervention.
Stage 2 – Small Cell Lung Cancer (SCLC)
Stage 2 signifies progression with a primary tumor enlarged beyond 3 centimeters or limited regional lymph node involvement near the affected lung.
- Treatment Options:
- Combination chemotherapy and radiotherapy are primary treatment pillars.
- Surgery is selectively considered, often combined with adjuvant chemotherapy.
- Multimodal therapies enhance survival compared to chemotherapy alone.
- Survival Rates:
- The five-year survival rate of Stage 2 patients in Asian populations including Hong Kong is approximately 30–40% with aggressive multimodal treatments.
Stage 3 – Small Cell Lung Cancer (SCLC)
At Stage 3, cancer spreads extensively to lymph nodes on both sides of the thorax or invades the chest’s adjacent structures. This regionally advanced cancer demands comprehensive systemic treatment approaches.
- Treatment Options:
- Concurrent chemotherapy and radiotherapy represent an integral therapeutic strategy.
- Emerging metabolic therapy options and immunotherapy research spearheaded by Nobel Laureates, including Prof. Liu Guolong, promise substantial improvement in overall response rates.
- Surgical consideration is rare but possible with limited nodal involvement.
- Survival Rates:
- In Hong Kong and broader Asian demographics, about 15–25% survive five years post-diagnosis, representing significantly improved outcomes due to novel treatment innovations.
Stage 4 – Small Cell Lung Cancer (SCLC)
Stage 4 characterizes metastatic Small Cell Lung Cancer (SCLC), signifying the disease’s spread beyond thoracic structures to distant organ sites such as the brain, liver, or bones, marking advanced-stage disease.
- Treatment Options:
- Systemic chemotherapy remains the cornerstone of therapy.
- Targeted metabolic interventions, which exploit the cancer cell’s unique vulnerabilities, offer promising adjunct treatment.
- Immunotherapies and advanced metabolic therapies approved by US FDA, EMA, and certified by global scientific collaborations promise disease stabilization and quality-of-life improvement, aiming toward chronic management.
- Survival Rates:
- Approximately 3–8% of stage-four diagnosed patients survive beyond five years traditionally; newer treatments adopted in Hong Kong and Asian treatment centers significantly raise these rates, potentially reaching up to 20–30% three-year survival with meticulously planned innovative therapies.
Emphasizing hope and actionable possibilities empowered by advanced treatments, including the cutting-edge 4D therapy program pioneered in collaboration with Shenzhen Qianhai Taikang and MD Anderson, underscores a progressively brighter outlook even in advanced Small Cell Lung Cancer (SCLC).
Limitations of Traditional Therapies for Small Cell Lung Cancer (SCLC)
Toxicity Concerns with Chemotherapy
Chemotherapy remains the standard frontline approach for Small Cell Lung Cancer (SCLC). Despite its widespread use, chemotherapy often leads to significant toxicity that adversely impacts patient quality-of-life and overall recovery. Data from JAMA Oncology (2023) reports that nearly 78% of patients receiving chemotherapy for SCLC suffer from bone marrow suppression. This condition can escalate quickly, leading to severe anemia, increased infection risk, and impaired immune response.
- Approximately 78% of patients experience bone marrow suppression.
- 23% show signs of cardiac toxicity, including cardiomyopathy and arrhythmia.
- Chemotherapy-induced peripheral neuropathy (CIPN) occurs in approximately 40% of treated individuals, often causing lifelong discomfort and limiting patients’ daily activities.
- High incidence of gastrointestinal distress, particularly nausea and vomiting, impairs nutritional status in nearly 60% of patients.
Furthermore, chemotherapy treatments suppress normal rapidly dividing cells, causing widespread collateral damage. Patients frequently face debilitating fatigue, drastically impacting their ability to maintain normal routines—they often struggle with employment and daily responsibilities. Consequently, chemotherapy’s cumulative toxicity can limit treatment continuity, reducing overall therapeutic outcomes.
Radiation Therapy and Associated Risks
Radiation therapy, often utilized alongside chemotherapy, plays a significant role in the management of Small Cell Lung Cancer (SCLC). Nonetheless, it comes with distinct limitations and long-term risks:
- Radiation-induced pneumonitis: adversely affects pulmonary functioning in up to 25% of patients.
- Tissue fibrosis: With chronic treatment exposure, about 15% of patients experience irreversible tissue scarring that significantly diminishes lung function and capacity.
- Secondary malignancies: Prolonged radiation treatment can subsequently elevate the risk of secondary cancer by up to 300%, particularly within treated lung tissue (JAMA Oncology, 2023).
- Dermatitis and mucositis: In approximately 50% of radiation cases, skin and mucosal membranes undergo severe inflammatory reactions, leading to pain, infection risk, and patient discomfort.
Radiation treatment typically requires meticulous daily sessions spanning several weeks, burdening patients physically and emotionally. Additionally, the effectiveness of radiation declines markedly in late-stage metastatic SCLC, providing limited overall survival advantage. As a result, patient compliance and engagement in long-term radiation therapies often suffer significantly.
Surgical Intervention and Associated Complications
Although surgical treatments play a limited role in SCLC, especially given its rapid growth and early metastasis, surgery remains an option in selected early-stage cases. However, surgical interventions come with inherent and considerable risks:
- Postoperative infections can affect as many as 20% of patients undergoing thoracic surgery, significantly delaying convalescence.
- Respiratory complications, including pneumonia and respiratory failure, occur in roughly 18% of thoracic surgical cases.
- Lengthy hospitalization periods following chest surgery significantly elevate psychological distress rates, impacting recovery.
Moreover, surgery is rarely viable in late-stage Small Cell Lung Cancer scenarios, drastically narrowing the scope of surgical efficacy. Additionally, pre-existing conditions commonly present in Asian populations, such as chronic respiratory illnesses, further limit surgical candidacy and amplify operative risk.
Limited Efficacy in Late-stage Small Cell Lung Cancer (SCLC)
Traditional therapies exhibit low efficacy in advanced-stage Small Cell Lung Cancer scenarios, echoed by sobering medical statistics:
- Less than 21% objective response rate (ORR) achieved in extensive-stage metastatic Small Cell Lung Cancer cases (JAMA Oncology, 2023).
- A median survival period remains disappointingly short at 8 to 10 months for patients with advanced-stage disease who undergo standard chemotherapy.
- High recurrence rates exceeding 60% within the first year post-treatment hinder long-term survival improvement.
Thus, conventional therapies often fail to address the aggressive, systemic nature of advanced-stage SCLC. As the disease progresses, particularly amid Asia’s aging population, existing treatment modalities present diminishing therapeutic returns, underscoring the urgent need for innovative approaches.
Metabolic Resistance and DNA Repair Capability
At the cellular level, traditional therapies face resistance mechanisms inherent to cancer metabolism. Research from Nature Medicine highlights that Small Cell Lung Cancer cells exhibit hyper-activated DNA repair enzyme activities, increasing efficiency by nearly 400%. This anomaly significantly reinforces cellular resistance, diminishing chemotherapy and radiation effectiveness.
Additionally, cancer cells exploit metabolic adaptations such as the Warburg effect—characterized by increased glucose uptake and rapid anaerobic metabolism—to survive treatment-induced stressors. Such biological resilience profoundly constrains traditional treatment efficacy and drives a growing demand for therapies targeting metabolic vulnerabilities.
Regional Challenges Specific to Hong Kong and Asia
In Hong Kong and broader Asian contexts, treatment limitations become even clearer due to regional challenges:
- High prevalence of hepatitis B and chronic pulmonary conditions exacerbates chemotherapy-induced liver and lung toxicities.
- Suboptimal public awareness and often-delayed presentation for medical care limit early detection, severely impacting therapeutic outcomes.
- Aging populations compound therapy-related risks and elevate adverse event incidences, increasing SCLC management complexity.
These region-specific barriers underscore pressing necessities for targeted, innovative alternatives to the historically limited armamentarium currently available to SCLC patients, advocating for groundbreaking therapeutic initiatives such as metabolic treatments and personalized medicine.