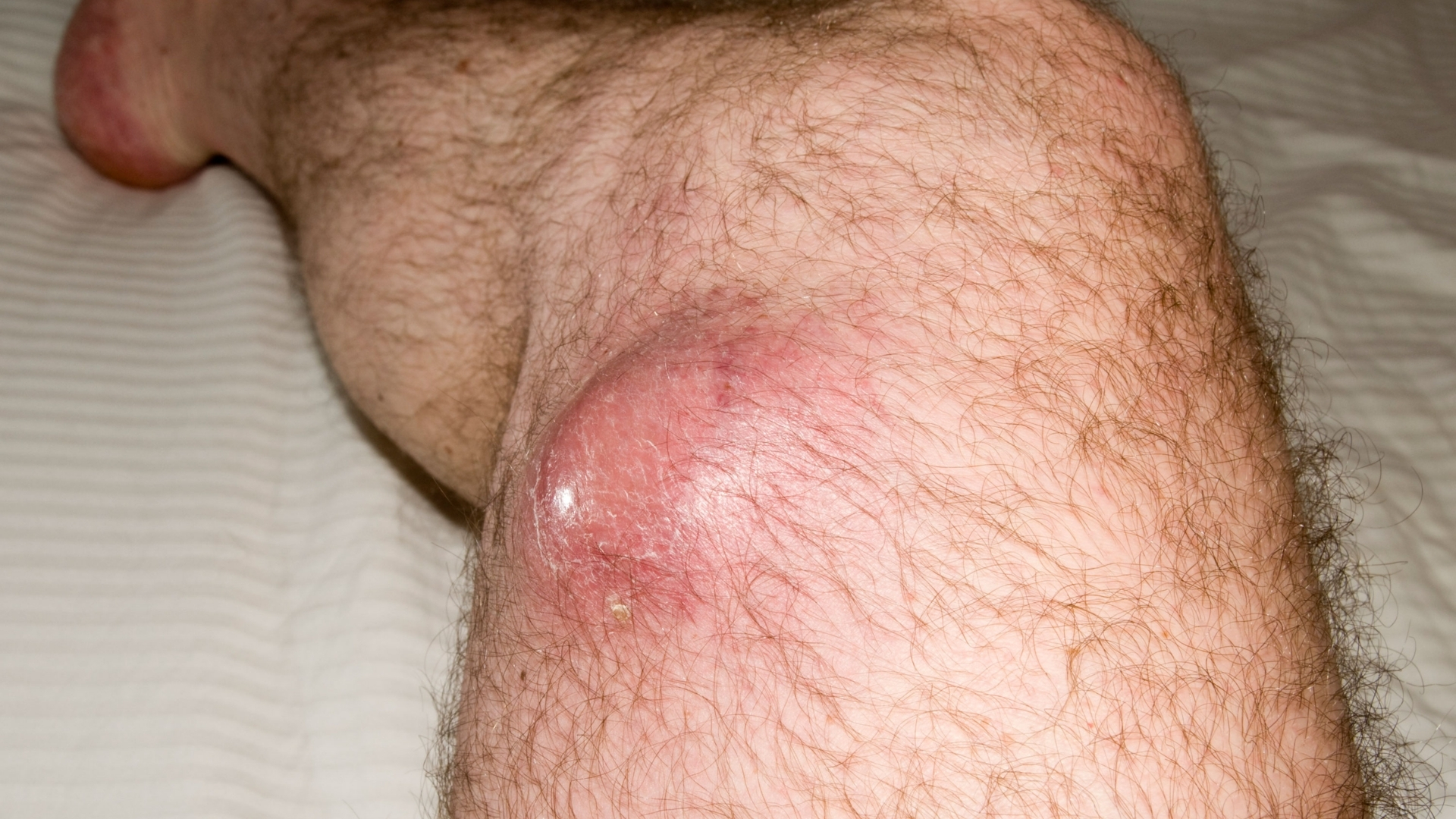
What Is Soft Tissue Sarcoma (STS)?
Soft Tissue Sarcoma (STS) encompasses a rare group of cancers originating in the soft tissues that connect, support, and surround other body structures. This type of cancer primarily affects muscles, tendons, fat, blood vessels, nerves, and other tissues, playing a crucial role in the body’s structural integrity. Understanding Soft Tissue Sarcoma (STS) requires attention to the biological complexities, including the notorious Warburg effect, where cancer cells consume glucose at up to 200x the rate of normal cells, fueling their rapid growth.
Globally, while not as prevalent as cancers like lung or breast cancer, Soft Tissue Sarcoma (STS) still presents significant challenges, affecting all age groups though predominantly found in older adults. According to WHO reports, the incidence of Soft Tissue Sarcoma (STS) is rising, with a noted prevalence in Asian populations, where unique genetic and environmental factors contribute to its development.
In Hong Kong and other parts of Asia, factors such as dietary habits, industrial pollution, and genetic predispositions may elevate risk. Patients often experience profound physical symptoms including fatigue and pain, compounded by an emotional landscape characterized by stress and anxiety.
To understand more about the cellular mechanics and structure of cancers, delve into our detailed cancer biology resource or explore our diagnostic approaches to catch Soft Tissue Sarcoma (STS) early.
Causes and Risk Factors of Soft Tissue Sarcoma (STS)
The causes of Soft Tissue Sarcoma (STS) are multifaceted, stemming from both intrinsic genetic mutations and extrinsic lifestyle and environmental factors. Certain genetic mutations, akin to BRCA1/2 mutations in breast cancer, are implicated though precise gene targets for Soft Tissue Sarcoma (STS) remain under investigation.
Environmental influences, including exposure to certain industrial chemicals and radiation, significantly increase risk. In Hong Kong, lifestyle factors such as obesity and high alcohol consumption are emerging as critical contributors. Importantly, metabolic vulnerabilities, specifically the reliance on glycolytic pathways for energy (glucose metabolism), enable cancer proliferation, mirroring the Warburg effect’s impact on other malignancies.
A unique concern within Asian populations includes the prevalence of hepatitis B, known to drive other cancers like liver cancer, which indirectly elevates risk for Soft Tissue Sarcoma (STS) through systemic inflammatory pathways.
Preventative measures, emphasizing regular screenings and lifestyle modifications (healthy diet, reduced alcohol intake), are essential. Early detection strategies can significantly improve outcomes and are encouraged as part of a proactive approach to health.
For an in-depth understanding of risk factors, visit authoritative sources like the World Health Organization or the National Cancer Institute.
Symptoms of Soft Tissue Sarcoma (STS)
Recognizing symptoms early is pivotal in managing Soft Tissue Sarcoma (STS). Symptoms can vary extensively, reflecting the heterogeneity of tumor biology. Below, we detail common STS-specific symptoms that emphasize the importance of early detection and timely intervention.
- Painless Lump or Mass: STS typically begins as an asymptomatic mass that grows gradually over weeks or months.
- Growth of Existing Mass: Rapid enlargement of a lump should prompt medical evaluation, reflecting aggressive tumor biology.
- Pain or Tenderness: Pain occurs less frequently in early STS but becomes more prevalent as tumors press against nerves or muscles.
- Swelling: Notable swelling in arms, legs, abdominal area, or back due to underlying tumor growth.
- Limited Mobility: Tumors near joints or muscles can cause stiffness or reduced movement.
- Systemic Symptoms: Rarely, advanced STS may prompt generalized symptoms like fatigue, unexplained weight loss, and fever indicating advanced metabolic burden.
- Neurological Symptoms: STS in certain tissues may cause neurological signs such as numbness, tingling sensations or even motor issues due to nerve involvement.
Symptom Variations by Stage
- Early Stage (Stages 1 & 2):
- Painless lump generally less than 5cm in diameter.
- Little to no discomfort or functional impairment, easy to overlook or misattribute.
- Advanced Stages (Stages 3 & 4):
- Significant enlargement of the mass, often more than 5cm.
- Increasing discomfort or severe pain often due to nerve compression or inflammation.
- Functional impairments leading to limited mobility, balance problems, or difficulty performing everyday tasks.
- Systemic symptoms like weight loss, chronic fatigue, or fever signaling metastatic spreads.
Tumor characteristics like rapid cell proliferation, invasive growth mechanisms, and enhanced glucose metabolism (Warburg effect, glucose consumption at rates 200x normal) directly influence these clinical signs. Therefore, awareness of early signs leads to prompt diagnosis and improved patient outcomes. For more detailed diagnostic approaches, please refer to our Soft Tissue Sarcoma (STS) Diagnostics page.
Stages of Soft Tissue Sarcoma (STS) and Survival Rates
Understanding the progression stages of Soft Tissue Sarcoma (STS) allows clearer insight into management pathways and their corresponding survival probabilities. The staging reflects the size, node involvement, and metastasis status, profoundly influencing patient prognosis and treatment flexibility.
Stage 1 – Soft Tissue Sarcoma (STS)
- Tumor typically localized, measuring 5cm or smaller.
- No regional lymph node involvement or distant metastasis.
- Highly amenable to surgical resection, complemented by precise radiotherapy or chemotherapy when necessary.
- Survival rates exceed 90% at five years post-diagnosis, particularly in metropolitan healthcare settings across Hong Kong and major Asian cities.
- Early detection significantly heightens cure likelihood, showcasing the benefit of regular screenings.
Stage 2 – Soft Tissue Sarcoma (STS)
- Tumors remain localized but exceed 5cm or show increasing biological aggressiveness.
- Potential evidence of mild local tissue invasion without nodal involvement.
- Strong recommendation for multimodal interventions combining surgery with radiation and advanced chemotherapy protocols.
- Five-year survival rates in Hong Kong data range between 70–85% depending on specific tumor histology and patient response to therapy.
Stage 3 – Soft Tissue Sarcoma (STS)
- Characterized by extensive local invasion, possibly compromising critical surrounding structures.
- Indicative regional lymph node involvement might be detectable.
- Multimodal treatment approaches become standard of care, integrating aggressive surgical interventions, chemotherapeutic regimens targeting metabolic vulnerabilities (e.g., glucose and glutamine dependence), and radiotherapy.
- The average five-year survival rate in definitive care centers across Asia ranges approximately from 50% to 70%, underscoring the urgency for early patient-focused interventions.
Stage 4 – Soft Tissue Sarcoma (STS)
- Tumors have metastasized to distant organs like lungs, liver, or bones, thereby presenting more complex therapeutic challenges.
- Treatment predominantly systemic, incorporating innovative metabolic therapies and targeted drug developments pioneered by Nobel-awarded researchers such as Allison and Semenza.
- Challenges arise due to adapted tumoral metabolic landscapes, underscoring therapies aimed at glucose metabolism interference and glutaminolysis inhibition.
- Although prognosis is guarded, recent advancements offer around 20–30% three-year survival rates according to Asian population-specific data, illuminating considerable possibilities for chronic disease management.
- Ongoing partnerships involving Shenzhen Qianhai Taikang, MD Anderson, and AllCancer emphasize the promise of transformative outcomes by turning STS into a manageable chronic health issue by 2025.
Early and accurate staging inherently impacts treatment effectiveness and survival outcomes. AllCancer, aligned with global standards, offers integrated diagnostic protocols and Nobel-backed metabolic innovations aimed at targeted interventions customized per patient stage. For more detailed treatment options, explore our dedicated Soft Tissue Sarcoma (STS) treatments page.
Diagnosis and Life Expectancy for Soft Tissue Sarcoma (STS)
Accurate diagnosis is pivotal in the management of Soft Tissue Sarcoma (STS). The process often begins with imaging techniques such as MRI, which provides detailed visualization of soft tissues, or PET-CT, which highlights metabolic activity characteristic of cancer cells, using the Warburg effect principle where cancer cells utilize glucose excessively. However, biopsy remains the definitive diagnostic tool. A core needle biopsy, for instance, is utilized to extract tissue samples for histopathological examination.
Advanced methods such as liquid biopsy are proving transformative. By analyzing circulating tumor DNA (ctDNA), this technique can identify mutations such as KRAS in up to 90% of certain cancers. Although primarily groundbreaking in lung cancer, its application is expanding in sarcoma research. These genetic insights are crucial for staging and determining prognosis, while also enhancing precision in treatment strategies.
Life expectancy for those with Soft Tissue Sarcoma (STS) hinges on several factors: the sarcoma’s stage at diagnosis, genetic characteristics of the tumor, and overall patient health. Early-stage sarcomas typically correspond with better prognoses. Furthermore, regular monitoring is essential for managing tumor evolution, employing both biochemical assays and imaging to identify changes promptly.
For further information on prognosis and diagnostics, the National Cancer Institute provides a comprehensive guide.
Treatment Options for Soft Tissue Sarcoma (STS)
The treatment landscape for Soft Tissue Sarcoma (STS) includes various conventional and innovative approaches. Surgery is often the first line of treatment. Depending on location and size, options can range from conservative resections to more extensive procedures. Surgery aims to achieve clean margins, minimizing the risk of recurrence.
Chemotherapy, though used variably, remains a cornerstone for certain sarcoma subtypes. Regimens may include agents like doxorubicin or ifosfamide. Meanwhile, radiation therapy capitalizes on targeted beams to destroy residual cancer cells post-surgery or to shrink tumors preoperatively.
Emerging within this domain are targeted therapies. These include tyrosine kinase inhibitors like imatinib for gastrointestinal stromal tumors, demonstrating promise in targeting specific molecular pathways.
Metabolic therapies are reshaping treatment paradigms by exploiting cancer-specific metabolic vulnerabilities. Strategies focusing on inhibiting glucose and glutamine consumption by targeting transporters such as GLUT1/3 are under exploration. These approaches aim to starve tumor cells of their primary energy sources, effectively hindering growth and proliferation.
The concept of personalized medicine is advancing. Genomic profiling of tumors facilitates customization of treatment protocols, catering to the unique genetic landscape of each cancer. In Hong Kong and broader Asia, medical choices are still evolving to incorporate these sophisticated technologies, though access can be challenging.
The potential of combining various modalities—surgical, chemotherapeutic, radiotherapeutic, and contemporary metabolic approaches—offers hope. The convergence of these treatments presents a powerful arsenal against Soft Tissue Sarcoma (STS), enhancing survival rates and quality of life.
Learn more about cutting-edge cancer therapies and how they are being applied to treat soft tissue sarcoma in our emerging cancer therapies page.
In conclusion, the diagnosis and treatment of Soft Tissue Sarcoma (STS) involve a multifaceted approach. Through precision diagnostics and personalized treatments, these complex malignancies are increasingly becoming manageable. There remains hope, as ongoing research continues to unveil promising therapies that may transform STS treatment paradigms and redefine patient prognoses.