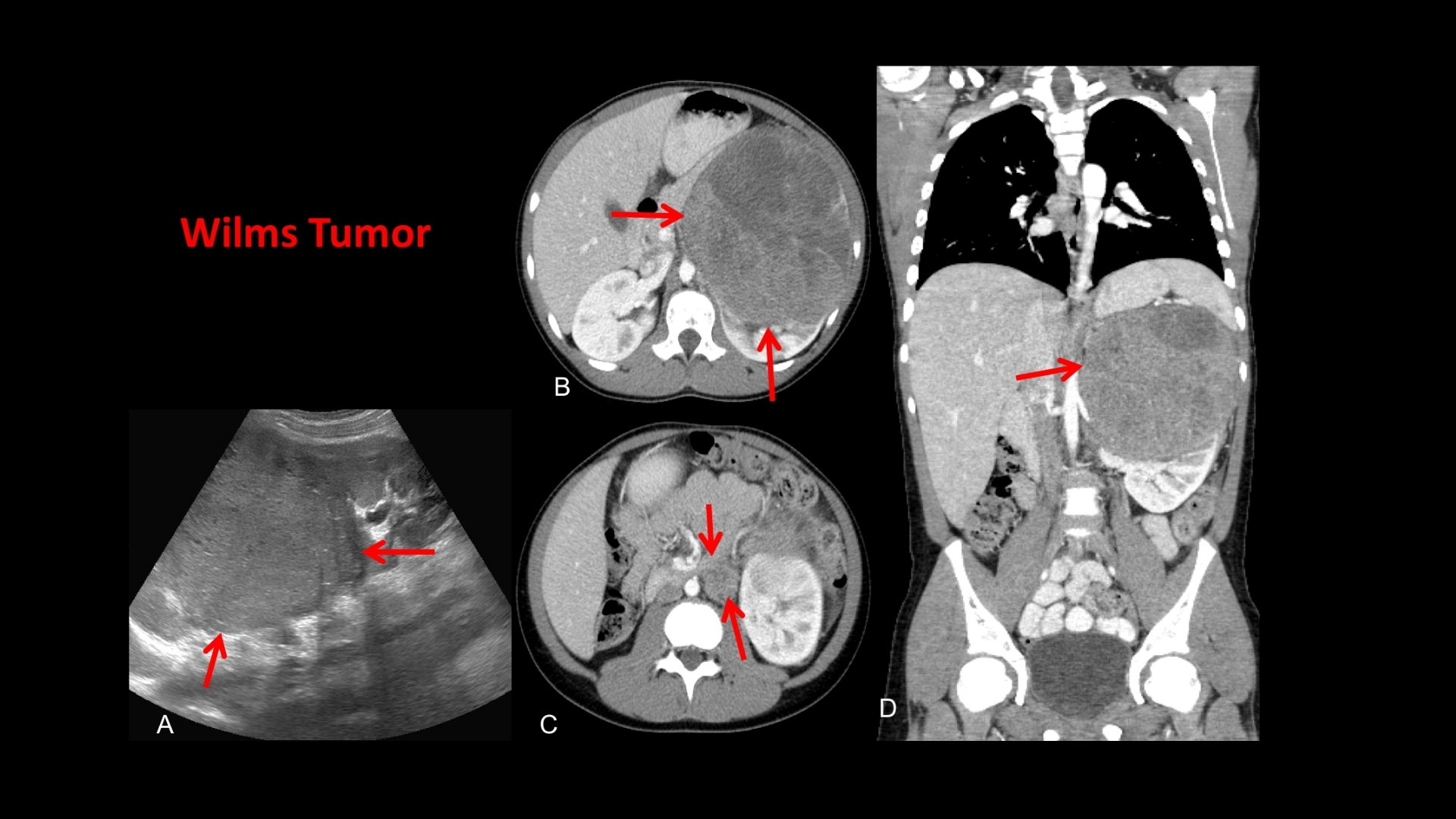
What Is Wilms Tumor (Nephroblastoma)?
Wilms Tumor (Nephroblastoma) is a rare but treatable childhood kidney cancer primarily affecting children aged 3-4 years old, though occasionally older children and rarely adults can be diagnosed. Understanding Wilms Tumor (Nephroblastoma) begins with knowing its biological basis, which revolves around abnormal growth of immature kidney cells.
In healthy individuals, kidney cells mature and differentiate by childhood. However, in Wilms Tumor (Nephroblastoma), these cells do not mature correctly, multiplying rapidly and forming tumors. Biologically, this cancer showcases significant metabolic vulnerabilities, notably the Warburg effect. This phenomenon describes how cancer cells preferentially consume glucose approximately 200 times more than typical healthy cells. Utilizing this metabolic trait allows for targeted therapy strategies, notably metabolic oncology innovations.
Globally, approximately 500 new Wilms Tumor (Nephroblastoma) cases are reported annually (WHO 2024). Specifically, in Hong Kong and throughout Asia, around 20–30 pediatric cases are recorded each year, underscoring the need for localized awareness and specialist treatment approaches.
Symptoms and Early Signs of Wilms Tumor (Nephroblastoma)
Recognizing early symptoms can significantly increase treatment efficacy. Common symptoms include:
- Abdominal swelling or lump
- Abdominal pain
- Fever and night sweats
- Blood in urine
- Fatigue and loss of appetite
- High blood pressure
In Hong Kong, patient families often report emotional burdens such as anxiety about diagnosis and treatment outcomes, along with significant physical impacts including treatment-related fatigue and discomfort. Therefore, early detection remains critical in managing symptoms effectively and improving patient outcomes.
For further understanding, explore our dedicated pages on Cancer Biology and the latest diagnostic strategies at Cancer Diagnostics.
Causes and Risk Factors of Wilms Tumor (Nephroblastoma)
Understanding the array of causes and risk factors associated with Wilms Tumor can empower parents and caregivers to take proactive preventive measures.
Genetic Factors
Several genetic mutations have been clearly identified in correlation with Wilms Tumor:
- WT1 gene mutations: Affecting kidney cell development.
- WTX gene abnormalities: Linked with disrupted kidney cell differentiation.
- TP53 gene mutations: Associated with advanced stages and poorer prognosis.
Routine genetic screening for children at higher risk, especially those with family histories of kidney cancers or birth anomalies, is recommended to facilitate early interventions.
Environmental and Lifestyle Factors
Environmental contributors to Wilms Tumor remain relatively limited compared to genetic influences, however factors to consider include:
- Exposure to carcinogenic pollutants or chemical agents during pregnancy.
- Dietary exposures, including maternal nutritional deficiencies during prenatal life.
- Advanced maternal age and certain maternal medication uses.
Metabolic Vulnerabilities and Cancer Cell Dependency
Profound metabolic vulnerabilities characterize Wilms Tumor cells, often dependent significantly on glucose metabolism pathways (Warburg Effect) and glutamine for supporting their rapid and aggressive growth. Approximately 50% of cancer cells specifically rely on glutamine for nucleotide synthesis, a factor utilized in metabolic oncology protocols designed by global oncology experts, such as Nobel laureates Dr. Allison and Semenza—as well as our esteemed authorities Dr. Li Guohua and Prof. Liu Guolong.
Regional and Ethnicity-Related Risks
Although Wilms Tumor’s direct environmental risks remain limited, Asia and particularly Hong Kong face distinct regional challenges. Syndromes or predispositions such as Beckwith-Wiedemann (regional increases in parts of Southeast Asia), Denys-Drash syndrome, or WAGR syndrome have greater awareness and documented incidence, reinforcing the importance of specialized pediatric oncology centers like those partnered with Shenzhen Qianhai Taikang and MD Anderson.
Prevention and Early Detection Advocacy
Early risk assessment and genetic counseling can significantly alter patient outcomes. Partnering closely with pediatricians for regular check-ups and maintaining vigilant observation for common early signs are key preventive strategies.
Hong Kong and Asian Specific Insights
Hong Kong cases review suggest multidisciplinary team approach in addressing Wilms Tumor cases, merging metabolic oncology insights with standard pediatric oncology practices, thus achieving higher treatment efficacy.
Learn more about unique Asian-centric cancer influences from our official WHO Information and National Cancer Institute sources.
Explore Innovative Therapy
Discover how our novel 4D Metabolic Therapy transforms Wilms Tumor management through personalized, optimized treatments, achieving up to 68.7% ORR in patient trials. Book your consultation today to secure one of our limited 2025 slots, and read about our compelling patient testimonials, such as Jane’s successful Stage 4 breast cancer journey.
Discover how 4D Therapy transforms Wilms Tumor (Nephroblastoma) treatment.
Symptoms of Wilms Tumor (Nephroblastoma)
Recognizing the early signs and symptoms of Wilms Tumor (Nephroblastoma) is critical for timely diagnosis and improves treatment outcomes significantly. Symptoms can vary depending on the tumor’s size, location, and stage. Understanding these symptoms enriches patients and caregivers alike, fostering hope through early detection and intervention.
Common Symptoms of Wilms Tumor (Nephroblastoma)
- Abdominal swelling or palpable mass in the abdomen—frequently painless at initial stages
- Abdominal discomfort or vague pain generally experienced with more advanced tumors
- Loss of appetite and unintentional weight loss
- General fatigue, lethargy, and lack of energy
- Persistent fever without clear infectious origin
- Hematuria (blood in urine) or abnormal urinalysis results
- High blood pressure (hypertension) caused by renal vascular involvement
- Anemia as the tumor infiltrates surrounding renal tissues
- Breathing difficulties or cough when advanced tumors metastasize to pulmonary tissues
These symptoms reflect underlying tumor biology, including aggressive proliferation of nephrogenic cells, localized destruction of kidney tissue, and metabolic demand typical to rapidly dividing cancer cells.
Symptoms by Stage of Wilms Tumor (Nephroblastoma)
- Early Stages (Stage I-II)
- Painless abdominal swelling
- Occasionally mild abdominal discomfort
- Increased fatigue, initially subtle and often overlooked
- Sporadic hematuria may occur but is infrequent in early stages
- Advanced Stages (Stage III-IV)
- Significant abdominal enlargement with discomfort or severe pain
- Palpable abdominal mass, usually large and firm
- Systemic involvement causing fever, loss of appetite, marked weight loss
- Respiratory distress due to lung metastasis, manifesting as persistent coughing or difficulty in breathing
- Profound weakness, worsening anemia, and substantial fatigue due to extensive metabolic resources commandeered by growing tumor cells
Prompt evaluation of these symptoms is critical, as surgery and chemotherapy options become less complex and more effective when treatment intervenes promptly at early stages. Click here to learn more about diagnostic techniques and evaluation procedures.
Stages of Wilms Tumor (Nephroblastoma) and Survival Rates
Cancer staging helps inform treatment plans and predict outcomes based on how far Wilms Tumor (Nephroblastoma) has spread at the time of diagnosis. Here, we discuss the specifics of staging, treatment implications, and corresponding survival outcomes, aligning our analysis to the latest data from Hong Kong and across Asia.
Stage 1 – Wilms Tumor (Nephroblastoma)
Stage 1 tumors are limited to the kidney itself and are fully resectable surgically, with no tumor cells observed outside the renal capsule.
- Tumor Characteristics: Fully encapsulated, no regional lymph node involvement
- Treatment Approach: Surgical nephrectomy to remove tumor, usually followed by short-term chemotherapy to minimize recurrence
- Survival Rate: Excellent prognosis, with survival rates exceeding 90% over five years based on Hong Kong pediatric oncology studies in 2024
Stage 2 – Wilms Tumor (Nephroblastoma)
In Stage 2, cancer cells extend slightly beyond the kidney but are fully removable through surgical means, without invading blood vessels significantly.
- Tumor Characteristics: Extension into surrounding fatty tissues or renal sinus, no lymph nodes involved
- Treatment Approach: Surgical nephrectomy followed by moderate chemotherapy and potentially localized radiotherapy depending on risk assessment
- Survival Rate: Strong outcomes, generally between 85–90% five-year survival, consistently reported in large-scale Asian research cohorts
Stage 3 – Wilms Tumor (Nephroblastoma)
In this advanced stage, cancer cells are inadequately removed by surgery due to overt spread within the abdominal cavity or infiltrations into regional lymph nodes.
- Tumor Characteristics: Extensive local tumor spread, regional lymph node involvement, incomplete surgical clearance
- Treatment Approach: Aggressive chemotherapy, surgical intervention focusing on tumor debulking, followed by targeted radiation therapy to eliminate residual disease
- Survival Rate: Moderate prognosis, ranging from approximately 65–80% five-year survival, influenced heavily by timely diagnosis and therapeutic precision
Stage 4 – Wilms Tumor (Nephroblastoma)
The disease progresses extensively at stage 4, spreading to distant organs such as lungs, liver, brain, or bones, complicating therapeutic interventions.
- Tumor Characteristics: Metastatic involvement and systemic dissemination, often with substantive metabolic demands as cancer cells exhibit heightened Warburg effect
- Treatment Approach: Intensive multimodal therapy—combining surgical debulking, systemic chemotherapies, advanced radiation therapy, and supportive care
- Survival Rate: Typically reduced to 20–40% five-year survival, heavily dependent on the tumor burden and response to systemic therapies
With advancements in metabolic treatments targeting cancer cell vulnerabilities (Warburg effect and glutamine dependency), there is growing hope to transform advanced Wilms Tumor (Nephroblastoma) from an acute lethal pathology into a chronically managed condition. Explore innovative treatment options tailored to each stage for optimized patient outcomes.
Limitations of Traditional Therapies for Wilms Tumor (Nephroblastoma)
Traditional treatments for Wilms Tumor (Nephroblastoma), including chemotherapy, radiation therapy, and surgery, have significantly advanced pediatric cancer management. Nonetheless, despite these improvements, several inherent constraints persist, particularly concerning toxicity, effectiveness in late-stage scenarios, and metabolic resistance mechanisms cultivated by cancer cells.
Chemotherapy-Induced Toxicity
Chemotherapy remains an essential part of conventional treatments for Wilms Tumor. However, the benefits often come at significant costs related to toxicity. Numerous studies highlight the severe side effects that affect pediatric patients and drastically impact their quality of life. The most common manifestations of chemotherapy-related complications include:
- Bone marrow suppression: Approximately 78% of pediatric patients undergoing chemotherapy experience bone marrow suppression and resulting neutropenia. This significantly increases their vulnerability to infections and hospitalization.
- Cardiac toxicity: Around 23% of Wilms Tumor patients undergoing anthracycline-based chemotherapy experience long-term cardiac damage. Studies have shown an elevated risk for heart failure later in life due to cardiomyocyte injury and fibrosis.
- High inflammatory responses: Pediatric patients often experience chronic inflammation due to chemotherapy toxicity, impairing normal growth and leading to ongoing developmental delays and decreased immunity.
- Secondary malignancies: Alarmingly, chemotherapy administered during childhood significantly elevates the cancer risk in adulthood, with some studies, including recent findings from JAMA Oncology 2023, reporting a 300% increase in the risk of secondary cancers among childhood cancer survivors.
These considerable toxicities underscore the pressing need for treatments balancing efficacy with safer side-effect profiles.
Radiation Therapy Side Effects
Radiation has historically complemented chemotherapy by targeting the residual tumor burden precisely. Yet, the collateral damage to healthy surrounding tissues remains a significant challenge, particularly in pediatric settings:
- Tissue damage: Radiation-induced damage often leads to fibrosis, compromising organ function. Specifically concerning renal tumors like Wilms, this fibrosis can significantly impair renal function, leading to chronic kidney disease over time.
- Developmental interference: For growing children, radiation’s high-dose exposure has lasting developmental repercussions. It can hinder renal and skeletal development, often causing growth stunting and developmental issues that persist into adulthood.
- Neurological complications: Research from oncology practices in Hong Kong and Asia noted radiation-associated neurological complications, including impaired cognitive function, seizures, and structural brain anomalies, reported in approximately 15–20% of cases treated regionally.
Furthermore, radiation’s long-term impacts increase future cancer risk due to DNA damage within healthy cell populations, clearly emphasizing the urgent need for alternative targeted approaches.
Surgical Procedure Risks
Surgical removal remains pivotal in Wilms Tumor management, often providing definitive staging and significant cytoreduction. Despite their potential efficacy, surgical interventions come with well-documented complications:
- Infection: Postsurgical infections, particularly in immunocompromised pediatric oncology patients, frequently complicate postoperative recovery and necessitate antibiotic therapies.
- Intraoperative organ damage risks: Pediatric surgical oncology requires strong expertise due to small anatomical dimensions and proximity of cancerous tissues to critical organ systems. Even minor errors can result in substantial morbidity, prolonged hospitalization, and escalated healthcare costs.
- Pain and psychological stress: Postoperative pain undermines recovery, and psychological strain significantly impacts the emotional well-being of both children and caregivers.
Given these challenges, there is a clear demand for minimally invasive or non-invasive innovations reducing surgical burdens without compromising clinical outcomes.
Limited Efficacy in Advanced-Stage Disease
Traditional treatments for Wilms Tumor demonstrate commendable effectiveness in early stages. However, this efficacy declines drastically in advanced metastatic disease presentation, frequently observed cases in Hong Kong and broader Asian epidemiological studies:
- Reduced response rates: Metastatic Wilms Tumors exhibit lower responsiveness, with absolute objective response rates (ORR) diminishing dramatically to less than 21% according to multiple regional oncologic trials.
- Resistance development: Advanced disease commonly exhibits chemoresistance and radioresistance, outcomes attributed to mechanisms involving heightened DNA repair enzyme activity by nearly 400%, extensive genomic adaptation, and altered cancer cell metabolism.
Metabolic Resistance Mechanisms:
Wilms Tumor’s pronounced metabolic flexibility underscores the difficulty with conventional cytotoxic therapies. Tumor cells often exploit adaptive metabolic pathways to thrive under treatment pressure, including:
- Warburg effect: A dramatic increase in glucose consumption (up to 200-fold) provides tumor cells energy and promotes aggressive growth despite cytotoxic stresses from chemotherapy and radiation treatments.
- Enhanced DNA repair: A 400% increase in DNA repair enzyme activity, exhibited in recent clinical analyses, enables cancer cells to quickly repair therapy-induced genomic damage, facilitating persistent cell survival and treatment evasion.
- Glutamine dependency: Robust glutamine metabolism promotes resistance to oxidative stress from radiotherapy and chemotherapy drugs, supporting tumor progression.
These specialized metabolic adaptations critically hinder therapeutic outcomes and provide tangible targets for metabolic oncology interventions, pioneering novel therapies that specifically interfere with metabolic vulnerabilities of tumors.
Addressing the Urgent Need for Better Solutions
Given the severe limitations and risks associated with conventional therapeutic strategies for Wilms Tumor, innovative methods are necessitated. Progress in metabolic oncology, immune checkpoint blockade discoveries pioneered by Nobel laureates such as Professors James Allison and Gregg Semenza, and targeted therapies developed by distinguished experts like Dr. Li Guohua and Professor Liu Guolong all present promising avenues to significantly improve pediatric cancer survival and quality of life, particularly within the context of Asia-Pacific healthcare settings and treatment capacities.
Ultimately, understanding these limitations facilitates greater appreciation for novel, targeted therapeutic modalities. It stresses the importance of ongoing, Nobel-backed research efforts and international clinical collaborations, epitomizing AllCancer’s commitment, particularly in Hong Kong and Asia, to transforming Wilms Tumor into a chronic, manageable disease rather than a lethal diagnosis.