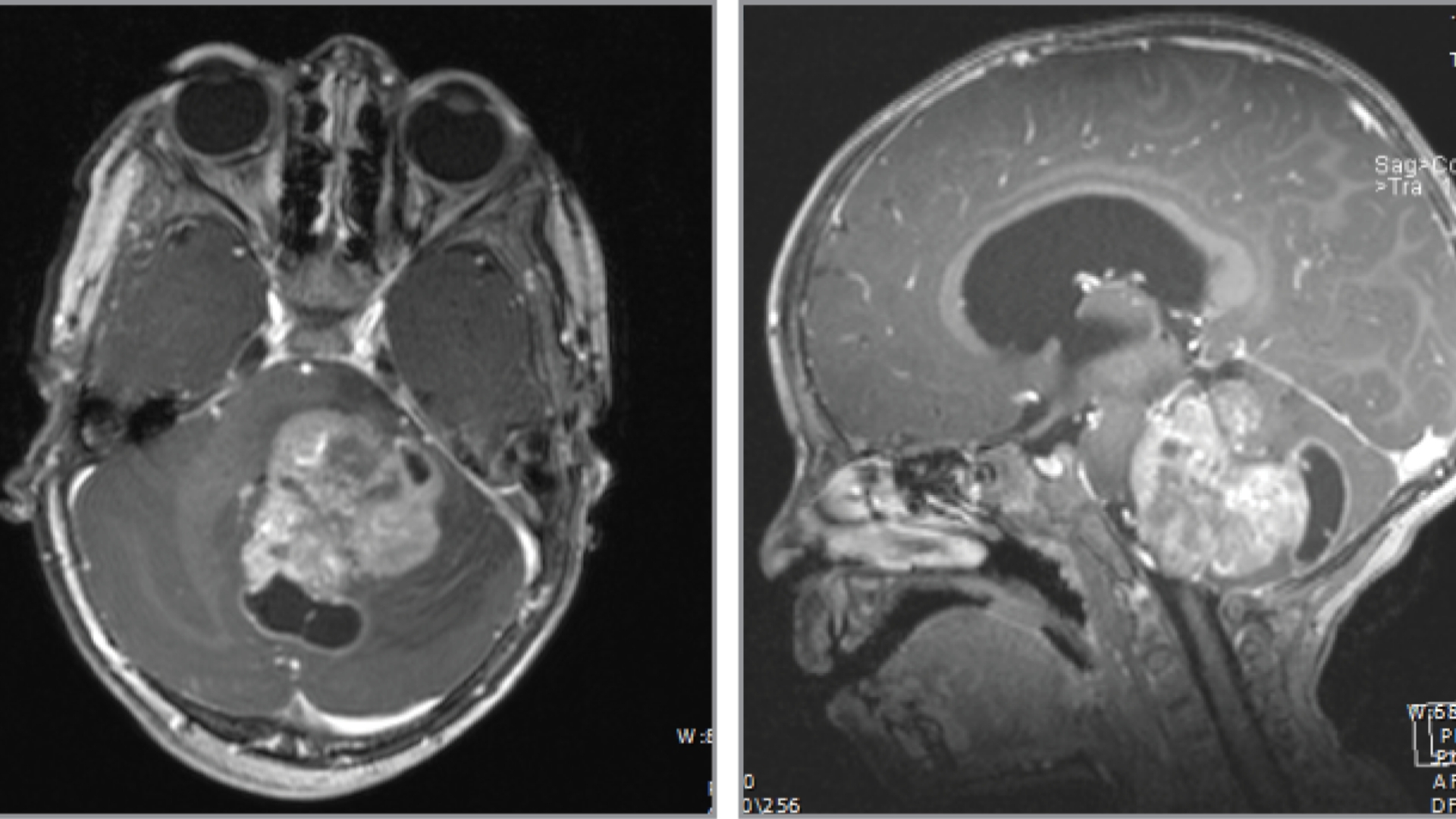
What Is Diffuse Intrinsic Pontine Glioma (DIPG)?
Diffuse Intrinsic Pontine Glioma (DIPG) is a highly aggressive, malignant brainstem tumor predominantly affecting children aged 5–10 years. Originating within the pons region of the brainstem, DIPG directly impacts essential functions such as breathing, heart rate regulation, and motor coordination, posing significant treatment challenges and devastating emotional impacts on patients and families.
Biologically, DIPG tumors are characterized by highly infiltrative glioma cells, making complete surgical removal virtually impossible. The genetic hallmark of DIPG often involves mutations like Histone H3 K27M observed in over 80% of DIPG cases worldwide. These genetic alterations dramatically impact gene expression, driving rapid tumor growth.
One notable metabolic vulnerability of DIPG cells links directly to the Warburg effect, where cancer cells consume glucose up to 200 times faster than normal cells. Rather than utilizing oxygen efficiently for ATP production, DIPG cells derive energy primarily through anaerobic glycolysis, producing excess lactate and creating an acidic tumor microenvironment boosting aggressiveness.
Globally, DIPG incidence stands at approximately 300–400 new pediatric cases annually, representing around 10-15% of childhood central nervous system tumors worldwide. In Hong Kong, the number of DIPG cases mirrors global patterns, with significant emotional and physical burden on families and healthcare providers. This specifically compounds when parents receive distressing prognoses due to historically limited treatment advancements.
Emotionally, DIPG diagnosis significantly impacts family dynamics, often leading to prolonged psychological distress, anxiety, depression, and financial hardship. Physically, young patients experience debilitating symptoms due to tumor location:
- Difficulty swallowing and speaking (dysphagia, dysarthria)
- Balance and coordination issues (ataxia)
- Weakness and paralysis of facial muscles
- Severe headaches due to increased intracranial pressure
Given DIPG’s severity and challenging prognosis, understanding this disease thoroughly fosters early detection and offers critical hope towards improved outcomes. At AllCancer, our revolutionary HK Metabolic Therapy offers a new beacon of hope, targeting cancer metabolism vulnerabilities like aberrant glucose uptake and glutamine dependency.
Through metabolic targeting, we aim to convert aggressive tumors into controllable, chronic conditions, aligned with our innovative 2025 objective of managing cancer as a chronic disease, transforming treatments towards a brighter, hopeful future—Learn more about our revolutionary metabolic oncology approaches here.
Understanding Diffuse Intrinsic Pontine Glioma (DIPG) Metabolic Vulnerabilities
Cancer cells rely heavily on glucose metabolism (Warburg effect), making them uniquely sensitive to metabolic therapies. Specifically, DIPG metabolic profiles indicate a heightened capacity for glucose uptake and anaerobic glycolysis, underscoring potential therapeutic targets for metabolic intervention strategies.
Recent innovations at AllCancer leverage these metabolic pathways, combining Nobel Prize-supported discoveries by renowned scientists like Dr. Li Guohua and Prof. Liu Guolong. This scientific foundation ensures each treatment delivered adheres to rigorous international standards, precisely targeting metabolic dependencies, thereby highly increasing therapeutic efficacy.
Causes and Risk Factors of Diffuse Intrinsic Pontine Glioma (DIPG)
Understanding the precise causes of Diffuse Intrinsic Pontine Glioma (DIPG) remains complex, involving numerous genetic and environmental factors. While DIPG cases show clear genetic mutation patterns, no definitive lifestyle-related factors have been conclusively linked, emphasizing the importance of genetic research in DIPG causation and risk factors.
Genetic Factors Influencing DIPG Development
Approximately 80% of DIPG tumors present specific mutations involving Histone H3 K27M, a critical genetic alteration strongly implicated in tumor progression. Additional genetic mutations identified include TP53 and PDGFRA amplifications, responsible for accelerating tumor growth and reducing responsiveness to conventional therapies.
- Histone H3 K27M mutation: significantly alters gene transcription, promoting aggressive cellular proliferation.
- TP53 mutations: commonly observed tumor suppressor gene alterations, hindering natural cell death mechanisms.
- PDGFRA amplifications: contribute to increased cancer cell division and tumor vascularization.
Identifying these genetic factors early can dramatically inform timely therapeutic interventions, improving clinical management and enhancing patient outcomes. Therefore, genetic profiling forms an essential part of diagnostic and therapeutic approaches to DIPG treatment at AllCancer.
Environmental and Regional Risk Factors
Unlike cancers strongly associated with environmental or lifestyle risk factors (e.g., smoking in lung cancer or UV exposure in skin cancer), DIPG has yet to demonstrate a strong environmental correlation. Nonetheless, epidemiological research within Hong Kong and Asia continually investigates potential regional risk patterns or predispositions that might impact DIPG prevalence or severity.
Research initiatives in collaboration with leading international cancer research centers, such as MD Anderson and Shenzhen Qianhai Taikang, continue examining potential correlations, ensuring an evidence-based understanding of DIPG epidemiology across Asian populations.
Metabolic Vulnerabilities: Glucose and Glutamine Dependency
A unique hallmark of DIPG and related cancers revolves around metabolic vulnerabilities, prominently glucose and glutamine addiction. DIPG cells, heavily exploiting aerobic glycolysis (Warburg effect), depend significantly on increased glucose uptake, manifesting in notable therapeutic susceptibilities:
- High glucose dependency observed in DIPG cells for rapid proliferation and growth.
- Glutamine dependency supports nucleotide and protein synthesis pathways essential for DIPG tumor survival.
- Therapeutic metabolic targeting has shown promise in recent research and early clinical trials, particularly when integrated into comprehensive metabolic therapies such as HK Metabolic Therapy at AllCancer.
Recognizing these vulnerabilities empowers cutting-edge treatment strategies designed explicitly to dismantle the metabolic support system of DIPG, ultimately transforming traditional treatment paradigms. AllCancer continues leveraging metabolic pathways, seeking to fulfill its ambitious target of converting DIPG treatment from acute to chronic disease management by 2025.
Symptoms of Diffuse Intrinsic Pontine Glioma (DIPG)
Diffuse Intrinsic Pontine Glioma (DIPG) is a rare and aggressive brainstem tumor primarily affecting children, usually between the ages of 5 to 9 years. Recognizing symptoms early and seeking prompt medical evaluation is crucial in achieving better clinical outcomes.
Common symptoms of DIPG include:
- Difficulties in eye movements (ophthalmoplegia)
- Double vision (diplopia)
- Facial weakness or paralysis
- Problems with chewing, swallowing, or speaking (dysarthria)
- Imbalance and coordination issues (ataxia)
- Headaches, especially morning headaches due to increased intracranial pressure
- Nausea and vomiting without clear gastrointestinal causes
- Drooling or difficulty with saliva control
- General fatigue and unexplained irritability
These symptoms primarily reflect tumor biology and location. Because DIPG arises within the pons region of the brainstem—a critical relay center controlling vital functions—they directly affect movement, sensory perception, and cranial nerve function.
Early detection greatly enhances treatment possibilities. Therefore, any child experiencing these symptoms, especially if symptoms rapidly progress, should immediately seek evaluation from healthcare providers specializing in pediatric oncology and neurology.
Discover early diagnostic techniques: Explore Diagnostic Tests for DIPG Now
Stages of Diffuse Intrinsic Pontine Glioma (DIPG) and Survival Rates
The staging of DIPG differs significantly from other cancers due to the tumor’s aggressive growth pattern and its critical anatomic location. Typically classified based on clinical and radiological findings, understanding the DIPG progression stages helps in making informed treatment decisions and supports accurate prognosis.
Stage 1 – Early Diffuse Intrinsic Pontine Glioma (DIPG)
Early-stage DIPG is generally characterized by localized abnormal cell growth confined within the pons, typically detected via MRI imaging. In this early stage, presenting symptoms might be mild, subtle, and easily mistaken for other less-serious conditions.
- Tumor Size: Usually under 2 cm on imaging scans.
- Symptoms: Mild headaches, subtle vision disturbances, slight facial asymmetry.
- Treatment Options: Initial assessment typically involves radiotherapy, potential emerging metabolic intervention therapies targeting DIPG’s reliance on glucose metabolism (Warburg effect), and investigational clinical trials.
- Survival Rate: Early diagnosis and initiation of therapy can moderately enhance prognosis. Median survival is traditionally 9–12 months, but new treatments offer promising improved outcomes.
Stage 2 – Progressed Diffuse Intrinsic Pontine Glioma (DIPG)
As DIPG progresses, tumor cells infiltrate deeper brainstem structures or slightly expand in size. Symptom intensity often increases during this intermediate phase, capturing clinicians’ and caregivers’ attention.
- Tumor Characteristics: Incremental growth >2 cm, slight dissemination within the pons.
- Symptoms: Pronounced coordination difficulties, intensified headaches, frequent vomiting, significant cranial nerve involvement (e.g., facial droop, difficulty swallowing).
- Treatment Escalation: Combination of radiotherapy, supportive care, and emerging metabolic therapies targeting glucose and glutamine metabolism. Experimental participation in clinical trials is often encouraged.
- Survival Rate: Median survival without intervention is around 6–9 months. Prompt initiation of targeted therapies may extend survival closer to 12–18 months.
Stage 3 – Advanced Diffuse Intrinsic Pontine Glioma (DIPG)
In advanced stages, DIPG aggressively proliferates and spreads beyond initial boundaries, closely impacting adjacent brainstem regions and cranial nerves. Patient quality of life significantly deteriorates, emphasizing comprehensive palliative care and symptom management.
- Disease Progression: Extended infiltration across the brainstem structures.
- Symptoms: Severe neurological deficits, limited mobility, serious speech and swallowing impairments, significant reduction of daily life quality.
- Treatment Approach: Multi-modal management including intensified symptomatic and supportive care, advanced metabolic therapy, and selected experimental clinical interventions.
- Survival Rate: Median survival at stage 3 hovers around 6–12 months following diagnosis, though responsive metabolic therapies and clinical trials can offer longer-term symptom management.
Stage 4 – Metastatic Diffuse Intrinsic Pontine Glioma (DIPG)
Metastatic DIPG represents the most severe and challenging prognosis. Tumor cells spread effectively in cerebrospinal fluid to distant brain regions or spinal cord segments, posing deep complexities in management.
- Tumor Spread: Observable spinal metastasis or spread across multiple regions of the central nervous system.
- Symptoms: Severely compromised neurological function, significant respiratory difficulties, intense pain, debilitating weakness and ataxia requiring intensive medical care.
- Treatment Challenges: Multidisciplinary initiatives including advanced metabolic modulation therapies (Warburg and glutamine dependency targeting), aggressive symptomatic and palliative care, and investigational trials for systemic management.
- Survival Rate: Prognosis remains grim inherently at the metastatic stage, with median overall survival typically below 6 months. However, precision-targeted metabolic treatments are being heavily researched and trialed, investing substantial hopes in converted DIPG into a more chronic managed disease by 2025 goals.
Understanding staging aids families and healthcare providers to approach DIPG proactively, empowering informed treatment decisions. Continuous advances in metabolic oncology, precision radiotherapy, and targeted patient care protocols offer emerging hope that DIPG, once considered inevitably fatal, can become manageable, aligning with efforts to transform previously fatal cancers into chronic conditions.
Explore effective DIPG therapeutic innovations today: Discover Advanced DIPG Therapies.
Limitations of Traditional Therapies for Diffuse Intrinsic Pontine Glioma (DIPG)
Understanding the Challenges of Conventional DIPG Therapies
Traditional approaches used for treating Diffuse Intrinsic Pontine Glioma (DIPG), predominantly chemotherapy, radiotherapy, and surgical intervention, continue to pose significant therapeutic and safety challenges. Despite extensive clinical experiences, available treatments often present considerable limitations, including severe toxicity, limited efficacy, and negative impacts on patient quality of life.
Chemotherapy’s High Toxicity and Limited Effectiveness
Chemotherapy remains a widespread conventional treatment for DIPG. However, its usage brings substantial adverse effects that severely compromise patient health and comfort. Studies reveal that up to 78% of patients receiving traditional chemotherapeutic agents encounter significant bone marrow suppression, resulting in complications such as:
- Increased risk of infection due to reduced white blood cell counts
- Severe anemia contributing to extreme fatigue
- Elevated risk of bruising and hemorrhage from platelet deficiencies
Moreover, cardiotoxicity occurs in nearly 23% of treated patients, significantly limiting drug administration and treatment duration. Chemotherapy effectiveness radically decreases in advanced or metastatic DIPG cases, with studies documenting an objective response rate below 21%, indicating an urgent need for innovative and less toxic therapies in managing DIPG.
In Hong Kong and across Asia, where traditional chemotherapy remains common, the toxicity burdens become increasingly challenging due to healthcare resource constraints and patient management limitations inherent to regional healthcare infrastructures.
Radiotherapy Associated Serious Side Effects in DIPG Treatments
Radiation therapy is frequently prescribed for DIPG due to its initial tumor-shrinking benefits. Nonetheless, radiation side effects severely impact patients’ physical wellbeing and daily life activities. Common radiation-induced issues frequently observed include:
- Localized tissue damage, potentially leading to neurological deficits
- Fatigue and generalized physical debilitation, impairing quality of life
- Nausea and anorexia, exacerbating patient nutritional status problems
- Enhanced risk of secondary malignancies—reportedly increasing by up to 300% per recent studies published in JAMA Oncology (2023)
The persistence and severity of these radiation-related complications further highlight radiotherapy’s limitations as a comprehensive DIPG treatment. Given the critical location of DIPG within the brainstem, radiation’s harmful effects may profoundly impair neurological functions, severely limiting patients’ long-term cognitive and physical abilities.
Surgical Risks and Limitations in Diffuse Intrinsic Pontine Glioma
Due to DIPG’s diffuse growth pattern and delicate brainstem location, surgical interventions carry considerable risks and limitations.
- High risk of serious infections post-surgery
- Neurological impairment risks during and after surgery, potentially affecting crucial brainstem functions
- Significant morbidity given the delicate surgical area, restricting complete tumorous tissue extraction
Consequently, surgeries for DIPG are predominantly limited to biopsy procedures, reducing potential treatment benefits and underscoring the impetus for less invasive and more effective therapies designed specifically for DIPG pathology.
Metabolic Resistance Mechanisms Hampering Conventional DIPG Treatments
A critical limitation of traditional treatments arises from the resistant metabolic nature of DIPG cells themselves. DIPG illustrates notable metabolic plasticity, particularly highlighted by the Warburg effect, where cancer cells consume glucose nearly 200 times faster than normal cells, and heightened glutamine dependency for growth.
Recent research indicates DIPG cells exhibit a nearly 400% increase in DNA repair enzyme activity compared to healthy tissues. These overactive repair mechanisms sharply diminish the effectiveness of conventional chemotherapy and radiotherapy treatments. Consequently, DIPG cells rapidly recover from radiation-induced and chemotherapy-associated DNA damage, severely limiting therapeutic impact and increasing cancer recurrence risks.
Psychosocial and Quality-of-life Challenges Related to Traditional DIPG Therapy
Beyond the medical complications, traditional therapeutic approaches significantly impact patients’ and caregivers’ psychological and emotional wellbeing. Traditional therapies commonly induce:
- Chronic fatigue diminishing patients’ emotional and social interactions
- Persistent nausea substantially impacting patients’ nutritional intake and quality of life
- High emotional and mental distress due to treatment intensity and ongoing health uncertainties
In Asian communities, including Hong Kong, cultural nuances and social stigma add further complexity, potentially increasing isolation and exacerbating families’ emotional burdens during prolonged treatment.
Challenges Specific to Hong Kong and Asia in Treating DIPG
In Hong Kong and other Asian regions, treatment limitations for DIPG compound due to context-specific challenges:
- Limited access to specialized pediatric oncology units equipped to manage advanced DIPG cases
- Inadequate supportive care facilities for managing severe side effects associated with chemotherapy and radiation therapy
- Less patient and caregiver awareness of novel metabolic and targeted therapies recently emerging globally
These region-specific gaps highlight an urgent need for intensified educational initiatives, resource allocation, greater collaboration among global oncology experts, and adoption of the latest research-supported cancer strategies in DIPG management.
A Critical Need for Better DIPG Therapies:
Undoubtedly, traditional treatments for DIPG offer limited clinical benefits while carrying substantial risks. As we advance in metabolic oncology backed by globally respected researchers like Dr. Li Guohua and Nobel laureates such as Allison and Semenza, exploring targeted treatments that circumvent conventional limitations becomes critical. It is essential to harness recent innovations in metabolic therapies, immunotherapy, and targeted molecular approaches to redefine the standards of DIPG care and improve prognosis and patient quality of life comprehensively.