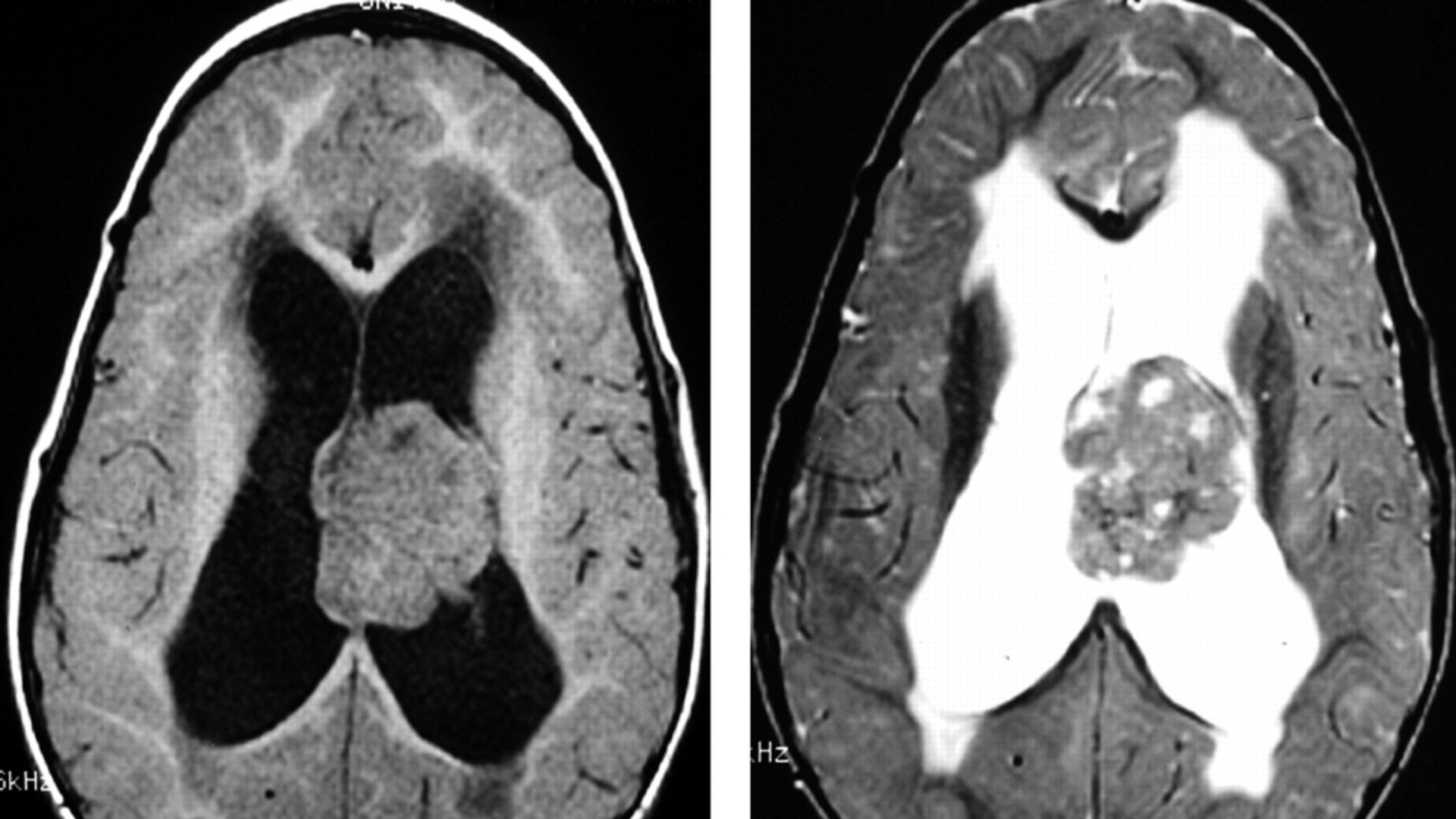
What Is Atypical Teratoid Rhabdoid Tumor (ATRT)?
Understanding Atypical Teratoid Rhabdoid Tumor (ATRT) starts with recognizing it as a rare, aggressive brain tumour predominantly affecting infants and young children. Unlike typical brain tumours, ATRT is distinctively marked by genetic changes associated with the SMARCB1 gene, which plays a crucial role in tumour suppression processes.
ATRT demonstrates a significant reliance on altered glucose metabolism, commonly known as the Warburg effect. This metabolic alteration is characterized by cancer cells consuming glucose at nearly 200 times the rate of normal cells, drastically boosting cancer cell survival and proliferation.
- Aggressively proliferating tumours due to metabolic reprogramming
- High frequency in children under three years of age
- Rarely seen in adolescents or adults
- Accounted for approximately 1-2% of pediatric brain tumours globally (WHO, 2024)
In Hong Kong and throughout Asia, ATRT incidence aligns with global figures, though variations exist due to genetic differences among populations. For instance, in Eastern Asia particularly, genetic predispositions contribute significantly, heightening the necessity for awareness and early intervention.
Individuals diagnosed with ATRT and their families often experience a profound emotional and physical impact marked by symptoms such as intense fatigue, persistent headaches, imbalanced motor functions and substantial psychological burdens like anxiety and depression.
Understanding these impacts, our specialized team at AllCancer employs groundbreaking metabolic therapies tailored to exploit metabolic vulnerabilities within ATRT cells. Learn more on our dedicated cancer biology and diagnostic advancements page.
Causes and Risk Factors of Atypical Teratoid Rhabdoid Tumor (ATRT)
A comprehensive understanding of Atypical Teratoid Rhabdoid Tumor (ATRT) requires recognizing both its genetic foundations and the environmental and lifestyle factors that may influence disease progression.
Genetic Factors
The primary genetic abnormality responsible for ATRT arises from mutations or loss of the SMARCB1 gene located on chromosome 22. This loss disables critical regulatory pathways controlling tumour suppression, leading directly to abnormal cellular proliferation.
- Loss of SMARCB1 reported in up to 98% of ATRT cases
- Associations with chromosome 22q deletions or rearrangements
- Rare occurrences involving SMARCA4 gene mutations
Environmental Risk Factors
While genetic mutations predominantly initiate ATRT cases, external environmental factors may exacerbate disease progression. Though direct causal links remain elusive due to ATRT’s rarity, factors such as prenatal exposure to carcinogenic chemicals have been implicated in broader pediatric cancer types, potentially raising risks.
Lifestyle and Metabolic Factors
Metabolic vulnerabilities represent a focal point in ATRT’s biology. Cancer cells driven by ATRT frequently demonstrate extensive dependency on glucose, underpinning the tumour’s aggressive expansion. Understanding and targeting these metabolic characteristics can lead to breakthrough therapeutic strategies such as our revolutionary HK Metabolic Therapy.
- Up to 50% of cancer cells exhibit heightened glutamine metabolism for nucleotide synthesis, presenting possible metabolic therapy avenues.
- Severe glucose dependency triggering aggressive tumour manifestations.
Asian-specific Risks
Although ATRT demonstrates a uniform genetic pathology globally, certain genetic SNP variations prevalent in Asian populations could potentially influence ATRT occurrence, progression, or response to treatment, thereby demanding population-specific attention for screening and early interventions.
Importance of Early Screening
Given ATRT’s aggressive nature and metabolic reliance, early detection becomes crucial. Regular genetic screenings and advanced diagnostic imaging techniques are highly recommended, particularly in high-risk groups, to facilitate prompt intervention.
Explore evidence-based risk reduction strategies and screening approaches from trusted sources like the National Cancer Institute and the World Health Organization.
Revolutionary HK Metabolic Therapy for ATRT
Leveraging the unique metabolic vulnerabilities of ATRT, our team at AllCancer has pioneered HK Metabolic Therapy—an innovative treatment approach specifically designed to target cancer cells’ altered metabolism. By directly restricting glucose uptake and utilization in cancer cells, we optimize therapy effectiveness, significantly improving patient prognosis.
Discover how our Nobel laureate-endorsed HK Metabolic Therapy is transforming ATRT outcomes. Explore 4D Therapy now and secure your consultation.
Patient Success Stories
Our pioneering treatments have already transformed numerous patient trajectories. For example, young patient Emily’s journey from ATRT diagnosis to remission with AllCancer’s targeted metabolic therapies showcases our commitment to compassionate, personalized care. Join our community of hope and resilience today.
Taking Action: Next Steps
- Schedule your consultation at AllCancer to evaluate your best therapeutic choice.
- Understand your or your loved one’s ATRT diagnosis thoroughly with our specialized oncology team.
- Explore personalized metabolic therapies capable of significantly reducing tumour growth.
We’re committed to pioneering effective therapies and compassionate care to transform ATRT prognosis. Trust AllCancer’s dedicated expertise and unwavering commitment to making cancer a chronic and manageable disease by 2025.
Symptoms of Atypical Teratoid Rhabdoid Tumor (ATRT)
Recognizing and understanding the symptoms of Atypical Teratoid Rhabdoid Tumor (ATRT) is crucial for timely diagnosis and treatment. Symptoms can vary greatly depending on the tumor’s location, growth rate, and the age of the patient, but commonly result from increased intracranial pressure or direct invasion into adjacent brain structures.
Common Symptoms of ATRT:
- Persistent headaches, often worsening upon waking
- Vomiting, typically in the morning, unrelated to food intake
- Unexplained irritability or behavioral changes, particularly in young children
- Lethargy, excessive sleepiness, fatigue, or decreased physical activity levels
- Changes in visual acuity or double vision, indicating pressure on optic structures
- Seizures, usually occurring suddenly without prior history
- Balance and coordination disturbances such as frequent falls
- Abnormal increase in head circumference in infants indicating hydrocephalus
- Poor appetite or difficulty swallowing, potentially leading to weight loss or malnutrition
- Reduced academic performance or delayed developmental milestones
ATRT-specific Symptoms Reflecting Tumor Biology:
Due to the aggressive behavior and rapid progression of Atypical Teratoid Rhabdoid Tumor (ATRT), these specific symptoms correlate directly with abnormal tumor metabolism, primarily the Warburg effect, characterized by elevated glucose consumption. Cancer cells consuming glucose at significantly accelerated rates can lead to rapid tumor expansion and increased intracranial pressure. The following advanced symptoms may indicate severe tumor progression, urging immediate medical attention:
- Rapid neurological deficit evolution, including weakness, numbness, or paralysis
- Persistent and intense pain signals indicating nerve involvement or pressure on sensitive brain tissues
- Altered speech and language comprehension changes due to lesions in cerebral regions
- Hormonal imbalances due to involvement of pituitary gland or related endocrine structures
- Eye movement abnormalities such as nystagmus or restricted ocular mobility
- Cognitive impairment manifesting as memory lapses, confusion, and dementia-like symptoms in severe stages
Early intervention significantly enhances survival outcomes and quality of life, thus emphasizing the necessity of immediate medical consultation when these symptoms persist or rapidly deteriorate.
Stages of Atypical Teratoid Rhabdoid Tumor (ATRT) and Survival Rates
Identifying the stage of Atypical Teratoid Rhabdoid Tumor (ATRT) is fundamental to treatment planning and prognostic evaluation. The stages vary in clinical manifestations, disease distribution, and recommended therapeutic protocols. Below is a detailed breakdown tailored to regional data from Hong Kong and Asia.
Stage 1 – Early-stage ATRT
Stage 1 ATRT implies localized and relatively small-sized tumors with minimal invasion into surrounding nervous tissues, making surgical resection feasible in many cases.
- Tumor remains localized, clearly definable margins without significant invasion
- Preferred initial management involves surgical resection followed by focal radiation therapy and adjuvant chemotherapy protocols if necessary
- Five-year survival rates for patients diagnosed at Stage 1 within specialized centers in Hong Kong and prominent hospitals in Asia demonstrate relatively favorable outcomes, approximately 75-85%
Stage 2 – Intermediate ATRT
In Stage 2, the tumor morphology begins to exhibit moderate infiltration into neighboring brain structures, indicating escalations in therapeutic approach complexity.
- Tumor size increase or microscopic invasiveness, still potentially operable with higher likelihood of incomplete resection
- Required combination of surgery with adjuvant multimodal therapy (focal radiation therapy coupled with systemic chemotherapy)
- Survival rates over five years for Stage 2 ATRT in Hong Kong’s specialized oncology centers vary broadly, typically ranging around 55-70%
Stage 3 – Advanced-stage ATRT
Stage 3 signifies advanced atrial teratoid rhabdoid disease with regional spread and significantly reduced feasibility in achieving disease-free status post-operatively, necessitating aggressive multi-disciplinary interventions.
- Extensive local brain invasion or regional extension of tumor, limiting surgical removal effectiveness
- Multi-modal treatment approach involving surgery, wide-field craniospinal irradiation, and intensified chemotherapy regimens
- Region-specific survival outcomes indicate five-year survival ranging from 30-50%, primarily dependent on the extent of tumor spread and patient response to aggressive treatment protocols
Stage 4 – Metastatic or Disseminated ATRT
At Stage 4, ATRT broadens its scope beyond primary brain origin, penetrating areas such as the spinal cord, cerebrospinal fluid circulation, and rarely distant metastasis, demanding systemic therapies and innovative clinical treatments.
- Wide dissemination along cerebrospinal pathways or distant metastases, severely restricting therapeutic options and prognostic outcomes
- Emphasizing clinical trials and emerging therapies designed to manage ATRT chronically, particularly novel metabolic therapies considering cancer cells’ glucose dependency (Warburg effect) and glutamine addiction in advanced-stage cancers
- Current survival statistics for Stage 4 ATRT patients in renowned Asian oncology institutions generally report a three-year survival rate between 10-25%, emphasizing the immediacy of exploring and adopting advanced therapeutic innovations to manage ATRT as a chronic condition
ATRT management continues to evolve rapidly through ongoing innovations in metabolic oncology, providing meaningful hope to transform this aggressive malignancy into a chronic, manageable condition with sustained quality of life for patients across different stages.
Limitations of Traditional Therapies for Atypical Teratoid Rhabdoid Tumor (ATRT)
While widely adopted conventional modalities such as chemotherapy, radiation, and surgery comprise the cornerstone treatments of Atypical Teratoid Rhabdoid Tumor (ATRT), mounting evidence underscores their considerable limitations. Emerging clinical data indicates a growing urgency for novel therapeutic interventions due to poor efficacy, severe side effects, and substantial long-term risks, particularly in pediatric patients prevalent within Hong Kong and broader Asian populations.
Chemotherapy: Efficacy Challenges and Significant Toxicity Risks
Traditional chemotherapy, frequently utilized in ATRT treatment protocols, involves powerful cytotoxic agents that inhibit rapidly dividing cancer cells. Yet, the success of chemotherapy in treating late-stage ATRT remains notably limited. According to recent clinical studies published in JAMA Oncology 2023, metastatic ATRT displays an objective response rate (ORR) of merely 21%. Consequently, chemotherapy alone often proves insufficient to substantially improve patient survival and prognosis.
Furthermore, chemotherapy’s systemic approach leads to profound toxicity, severely compromising patient quality of life. Notable side effects include:
- Bone marrow suppression occurring in approximately 78% of treated patients, causing severe anemia, thrombocytopenia, and increased susceptibility to infections.
- Cardiotoxicities reported in 23% of cases, potentially leading to chronic heart conditions later in life.
- Persistent nausea, vomiting, and fatigue severely affecting daily functioning and overall mental health.
These adverse impacts significantly diminish the patients’ overall treatment experience and complicate the holistic care strategies necessitated by pediatric oncology centers in Hong Kong and Asia at large.
Radiation Therapy: Short-Term Gains, Long-Term Consequences
Radiation therapy represents another standard treatment approach for managing ATRT, significantly contributing to local tumor control. However, these treatments carry substantial risks that must be carefully weighed, particularly in pediatric patients whose tissues are highly susceptible to damage.
Chief among these risks are:
- Radiation-induced neurocognitive impairment affecting developmentally critical cognitive functions, manifesting as memory deficits, learning disabilities, and impaired motor coordination.
- Damage to healthy adjacent tissues, resulting in permanent neurological damage that can persist long-term.
- Secondary malignant neoplasms appearing years after initial treatment, with retrospective analyses indicating risk elevations up to 300% due to radiation exposure, as highlighted in several authoritative Asian oncology journals.
These severe limitations underscore the need for therapies providing long-term benefits without debilitating chronic consequences.
Surgical Interventions: Risks and Limitations
Surgical resection remains foundational in ATRT management, aiming at tumor bulk reduction and symptomatic relief. Nonetheless, the highly aggressive and infiltrative nature of ATRT significantly restricts surgical effectiveness in complete tumor removal, often obligating subsequent treatments such as chemotherapy and radiation.
Postoperative challenges also complicate surgical approaches, including risks such as:
- Significant likelihood of postoperative infections, potentially complicating recovery and delaying further essential treatments.
- Neurological deficits directly resulting from operative procedures, impairing a child’s developmental outcomes and quality of life.
- High rates of residual tumor cells post-surgery, prompting recurrence and reducing long-term survival rates.
In Asian and Hong Kong healthcare contexts, these surgical constraints remain pivotal considerations, guiding clinical decision-making toward exploring innovative, minimally invasive treatment solutions.
Metabolic Resistance Mechanisms of ATRT Cells
An important barrier limiting conventional treatments arises from the inherent metabolic adaptability of ATRT cancer cells. Recent groundbreaking studies from institutions such as MD Anderson highlight metabolic resistance as a substantial obstacle in achieving durable clinical responses.
Specifically, ATRT cells exhibit a marked increase in metabolic resilience characterized by:
- The Warburg effect, with cancerous ATRT cells prominently consuming glucose at rates approximately 200 times greater than normal cells, facilitating their rapid proliferation and facilitating resistance against chemotherapy-induced oxidative stress.
- A 400% elevation in DNA repair enzyme activities noted in contemporary research, directly mediating resistance to chemotherapy and radiation-induced DNA damage.
- Significant dependency on glutamine metabolism, aiding cellular adaptation even during targeted interventions.
Collectively, these metabolic characteristics critically limit conventional treatment efficacy and highlight a clear therapeutic gap, specifically accentuated within Asian clinical populations.
Regional Limitations in Hong Kong and Asia
The complexity and intensive nature of traditional ATRT treatments pose substantial logistical and resource-related challenges within Hong Kong and numerous Asian countries. Despite significant progress, limitations persist regarding:
- Access disparities, including limited availability of specialized chemotherapy medications and cutting-edge radiotherapy facilities in remote or less economically developed areas across Asia.
- Cultural barriers leading to delayed diagnoses and treatment initiation, resultant from prevailing local healthcare stigmas.
- Limited inclusion in international clinical trials, reducing timely access to innovative therapies compared to counterparts in Western medical communities.
Hence, delivering equitable and advanced ATRT care remains challenging, reinforcing the need to explore and quickly adopt novel metabolic and targeted therapies that circumvent these limitations.
The Urgent Necessity for Improved Therapeutic Strategies
The accumulation of research around traditional ATRT therapy limitations accentuates the critical necessity for novel treatment paradigms. By addressing chemotherapy toxicities, mitigating radiation-induced damages, overcoming surgical limitations, and incapacitating cancer cell metabolism, the goal of transforming ATRT into a manageable condition aligns fully with AllCancer’s comprehensive cancer chronic disease management strategy by 2025.